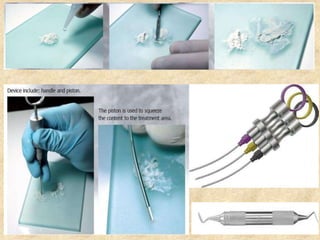
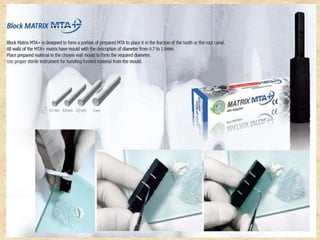
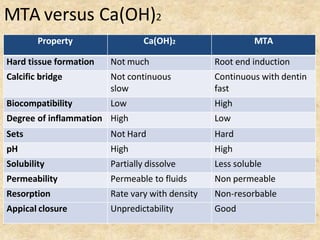
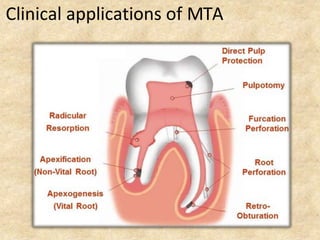
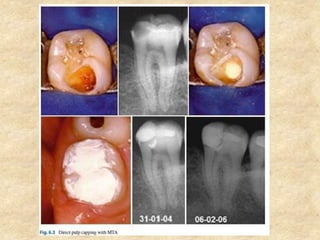
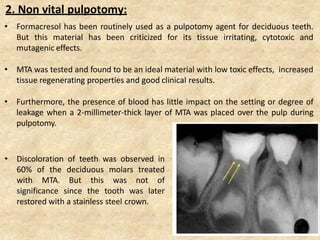
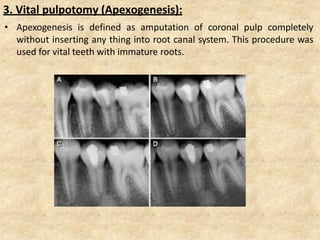
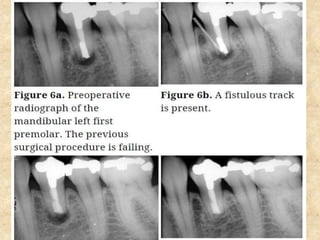
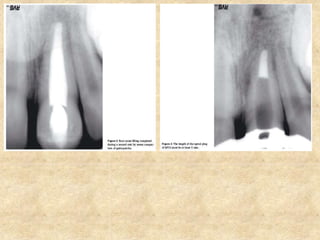
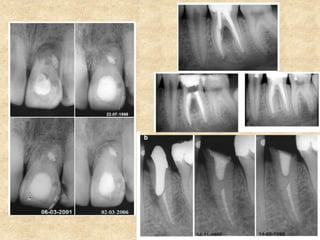
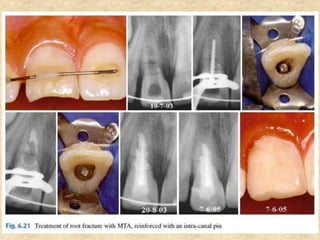
MTA was introduced in 1993 and approved by the FDA in 1998. It exhibits good biocompatibility, sealing ability, and tissue regeneration. MTA is available as grey or white powders mixed with water. It has a high pH and compressive strength increases over time. MTA has applications in pulp capping and non-vital pulpotomies due to its biocompatibility and ability to stimulate hard tissue formation and repair.