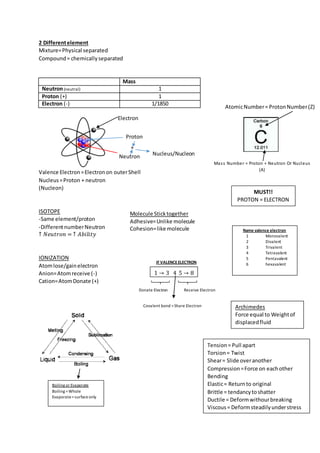
This document covers various physics concepts including units of measurement, properties of matter, forces and motion, heat and temperature conversions. It defines important terms like density, power, strain, and specific gravity. Key concepts about atoms are described such as atomic number, mass number, isotopes, ionization, and the structure of the nucleus.