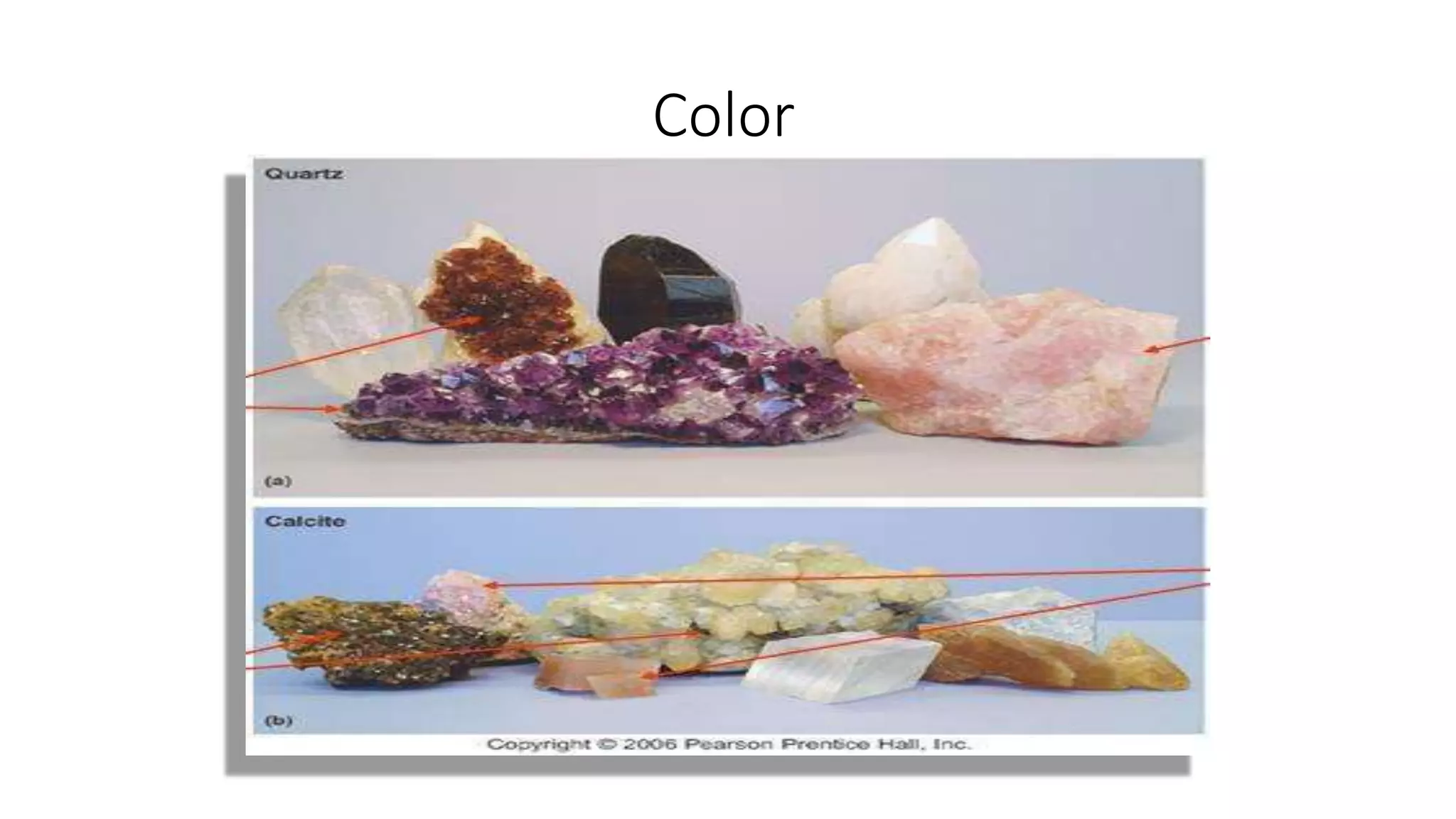
The document discusses minerals and their properties. It defines a mineral as a naturally occurring, homogeneous, solid substance with a defined chemical composition and internal atomic structure. Minerals are building blocks of rock. The study of minerals is called mineralogy. There are over 4,900 known mineral species. Minerals form through igneous, sedimentary and metamorphic processes. Their identification is important for civil engineering applications when evaluating rock properties. Minerals can be identified by their physical properties like color, streak, luster, hardness, crystal structure, cleavage and density, as well as their chemical composition.