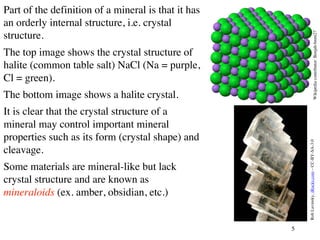
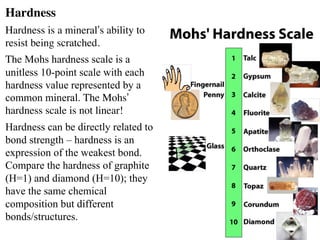
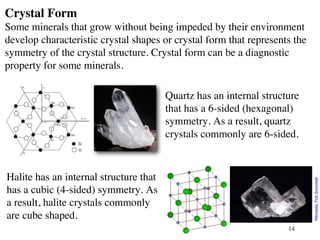
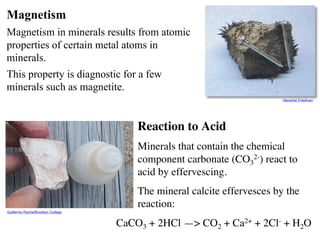
The document provides an overview of minerals, presenting definitions, classifications, and diagnostic properties. It explains that minerals are naturally occurring inorganic solids with a distinct chemical composition, and highlights various common minerals along with their characteristics. Key identification properties include color, streak, luster, hardness, and crystal structure, with examples illustrating their significance in geology and industry.