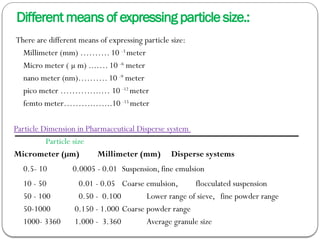
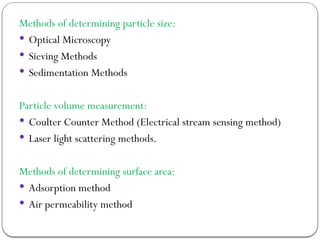
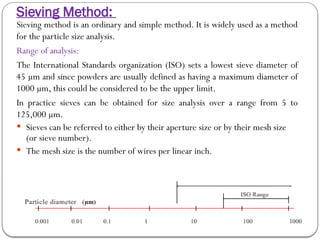
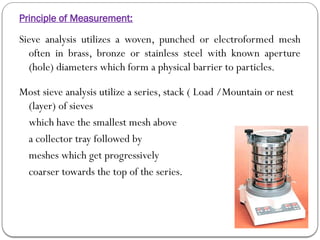
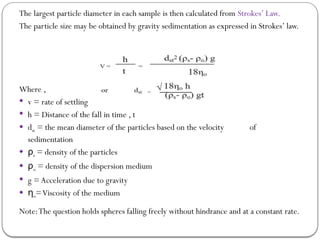
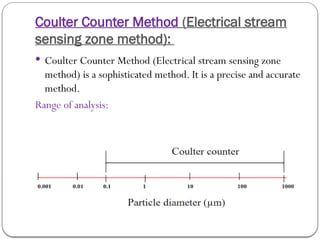
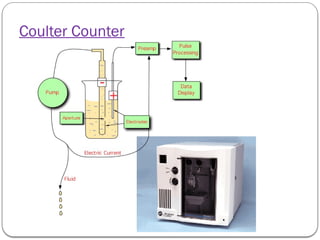
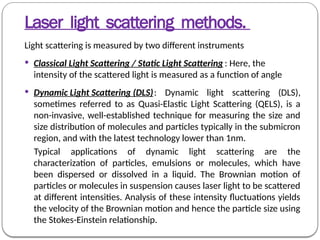
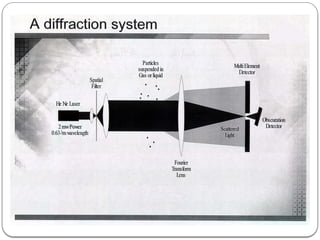
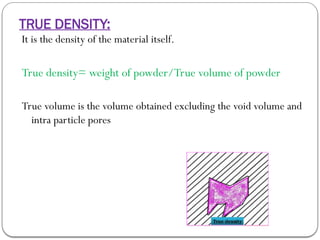
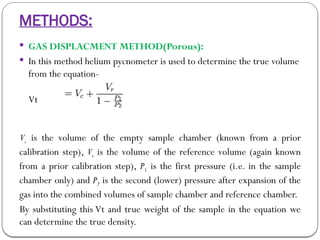
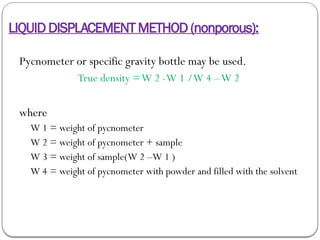
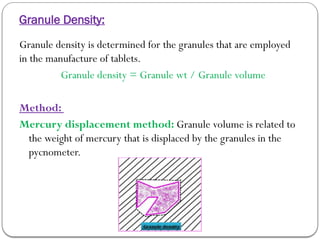
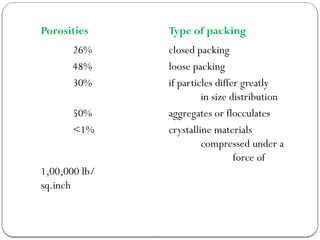
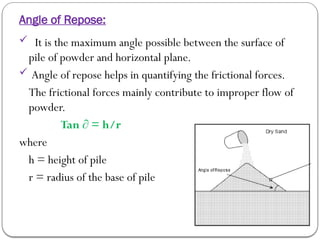
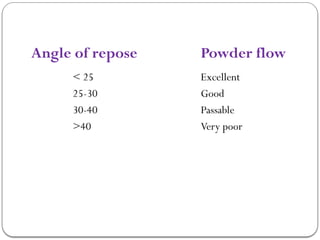
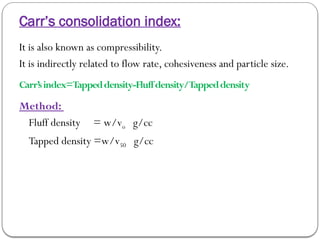
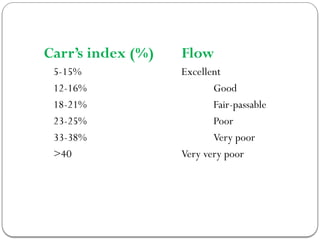
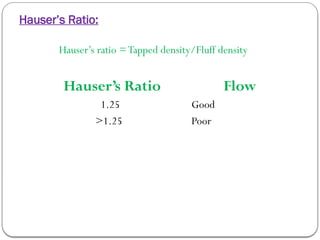
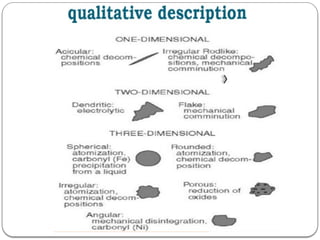
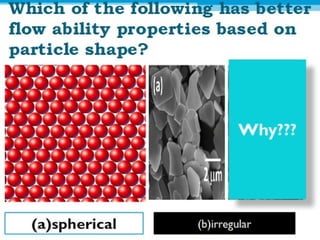
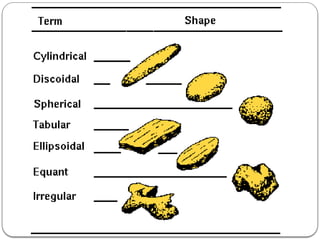
Micromeritics is the science of small particles, focusing on particle size, size distribution, shape, surface area, and pore size, which are crucial in pharmaceutical applications. Understanding micromeritics impacts drug release, stability, mixing properties, and ultimately the effectiveness of dosage forms. Various methods are employed to determine particle size and characteristics, including sieving, sedimentation, and laser light scattering, with relevance to drug absorption and safety.