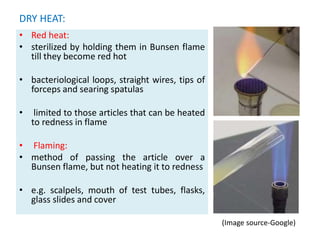
This document defines key terms related to sterilization and disinfection. It discusses various physical and chemical methods for sterilizing and disinfecting, including heat, radiation, filtration, and chemicals. Heat-based methods include moist heat using steam or boiling water and dry heat using ovens or flames. Radiation includes UV and ionizing radiation. Filtration separates microbes from liquids using various filter types. Common chemical disinfectants discussed are phenols, halogens, alcohols, aldehydes, and heavy metals. The ideal properties and mechanisms of action for disinfectants are also summarized.