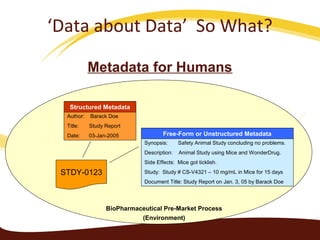
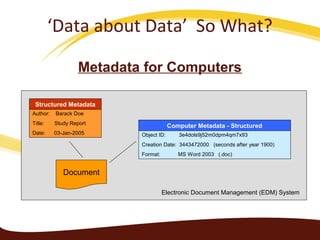
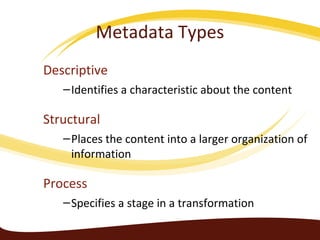
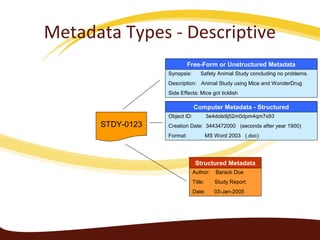
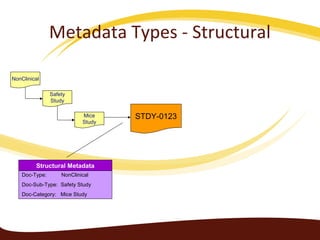
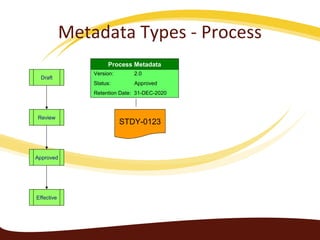
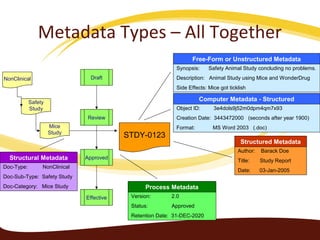
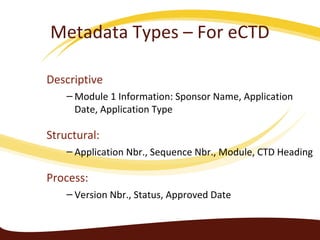
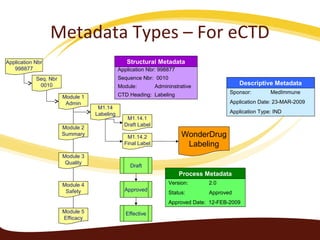
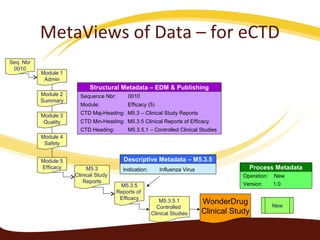
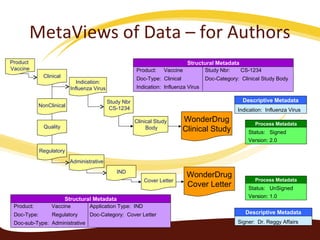
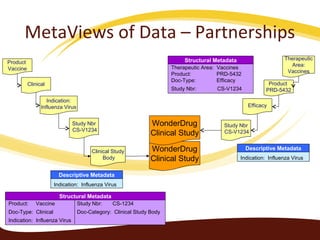
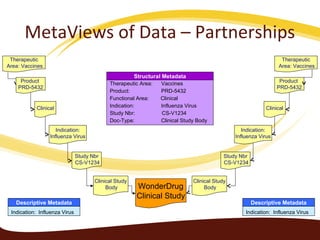
Metadata can be used for humans or computers. Humans use descriptive metadata while computers use structured metadata. There are different types of metadata including descriptive, structural, and process metadata. Electronic document management systems can provide different views or "metaviews" of data based on the metadata used. Standardizing metadata is important to avoid issues when different systems use inconsistent metadata, which can waste resources.