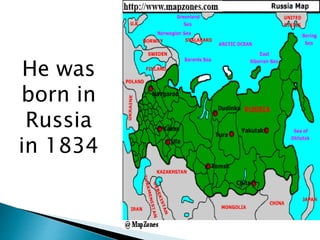
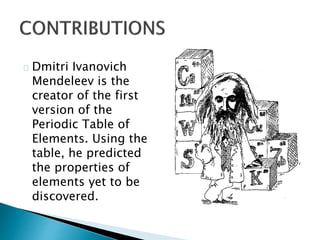
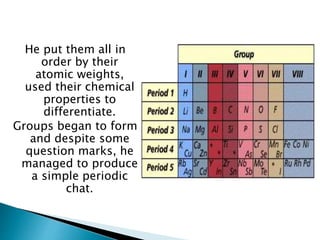
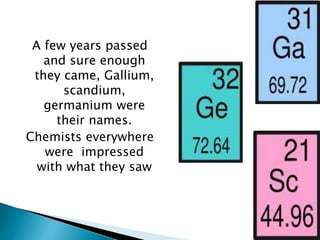
Dmitri Mendeleev was a Russian chemist born in 1834. He studied chemistry and graduated from St. Petersburg University in 1856. After graduating, he became a professor and was considered one of the greatest chemistry teachers of his time. Mendeleev is most famous for creating the first version of the periodic table of elements in 1869, in which he arranged the elements based on their atomic weights and properties. Using his periodic table, he was able to predict the properties of elements that had not yet been discovered. The periodic table became widely accepted among chemists and helped advance the field of chemistry. Mendeleev received several honors for his work, including the Copley Medal, and element 101 was named Mende













![Classification: is an actinide metal
Atomic weight: 258
State: solid
Melting point: 827 oC, 1100 K
Electrons: 101
Protons: 101
isotope: 157
Electron shells: 2,8,18,32,31,8,2
Electron configuration: [Rn] 5f12 7s2](https://image.slidesharecdn.com/newmicrosoftofficepowerpointpresentation2-140725113317-phpapp01/85/MENDELEEV-18-320.jpg)

