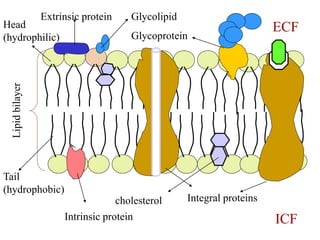
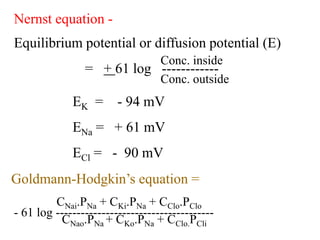
This document discusses the structure and functions of the cell membrane. It is composed of a phospholipid bilayer with integral and peripheral proteins. The membrane regulates the movement of substances via passive and active transport mechanisms. Passive transport includes simple diffusion, facilitated diffusion, filtration, and osmosis which move substances down their concentration gradients. Active transport requires energy and transports substances against their gradients using pumps like the sodium-potassium pump.