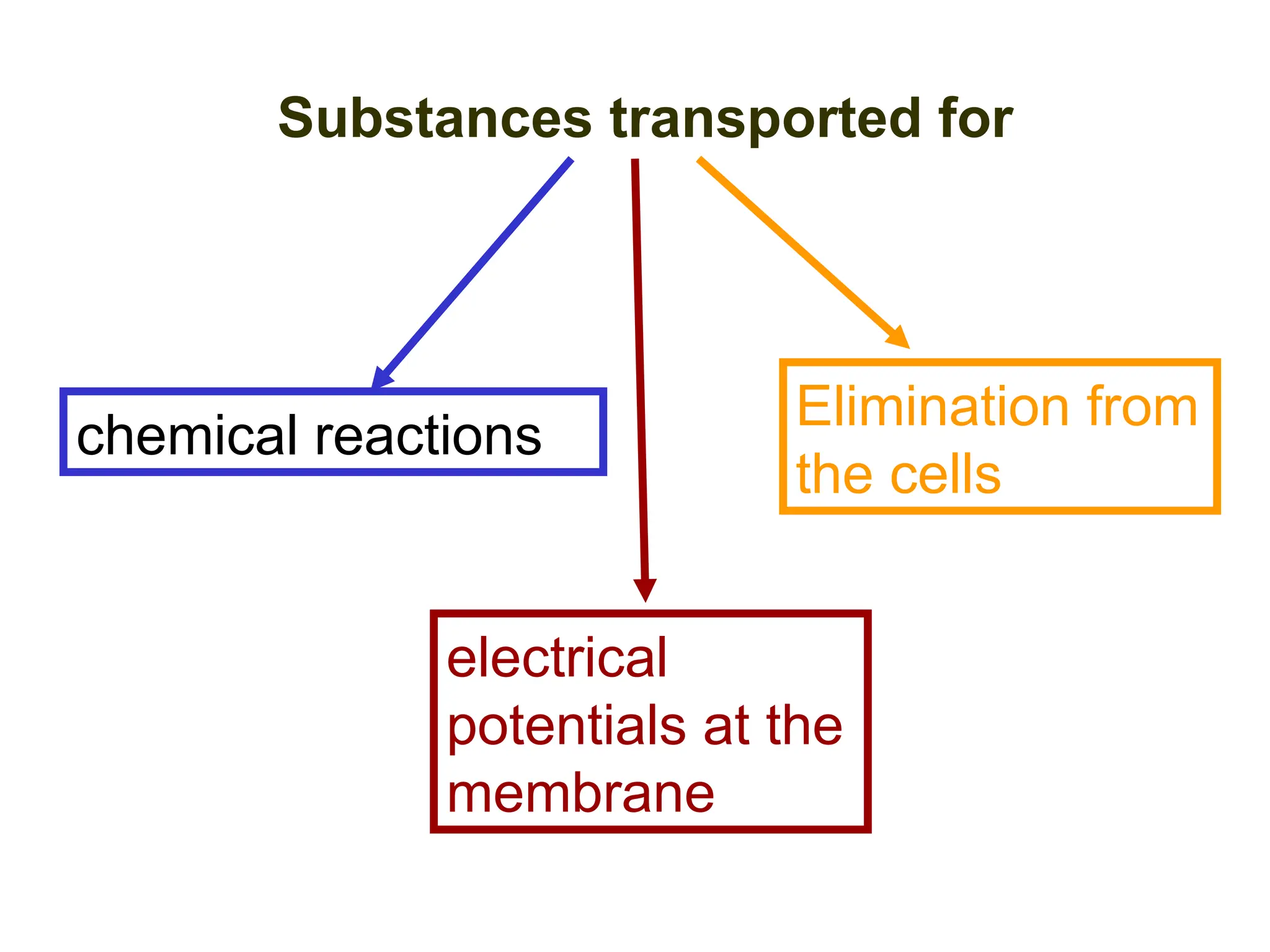
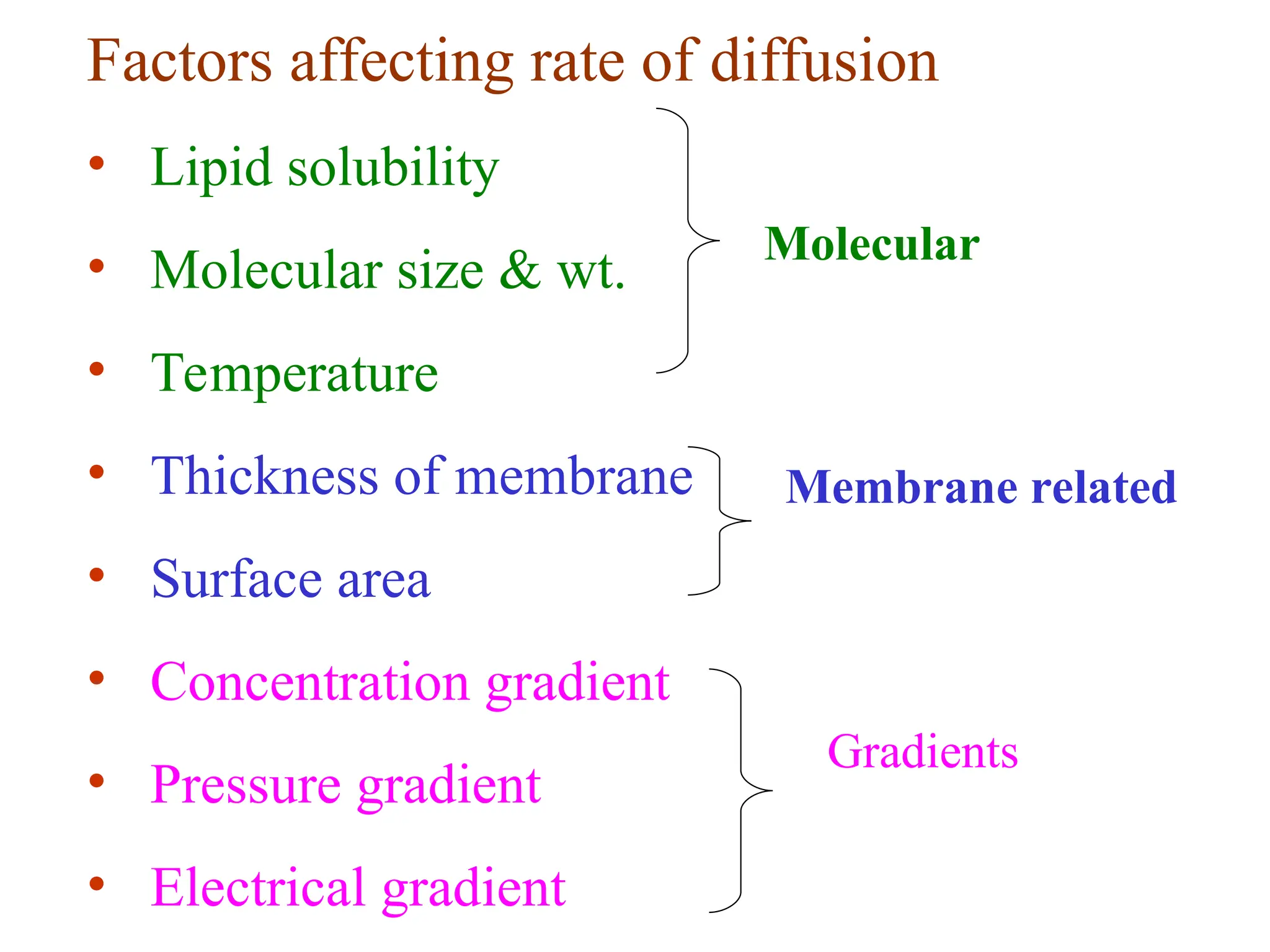
The document discusses the structure and function of cell membranes, detailing the composition, transport mechanisms (both passive and active), and the processes of osmosis, diffusion, and filtration. It highlights various transport methods, including simple diffusion, facilitated diffusion, primary and secondary active transport, as well as endocytosis and exocytosis, emphasizing the role of ATP and specific carrier proteins. Additionally, it covers the physiological principles governing these transport mechanisms, such as concentration gradients and osmotic pressure.