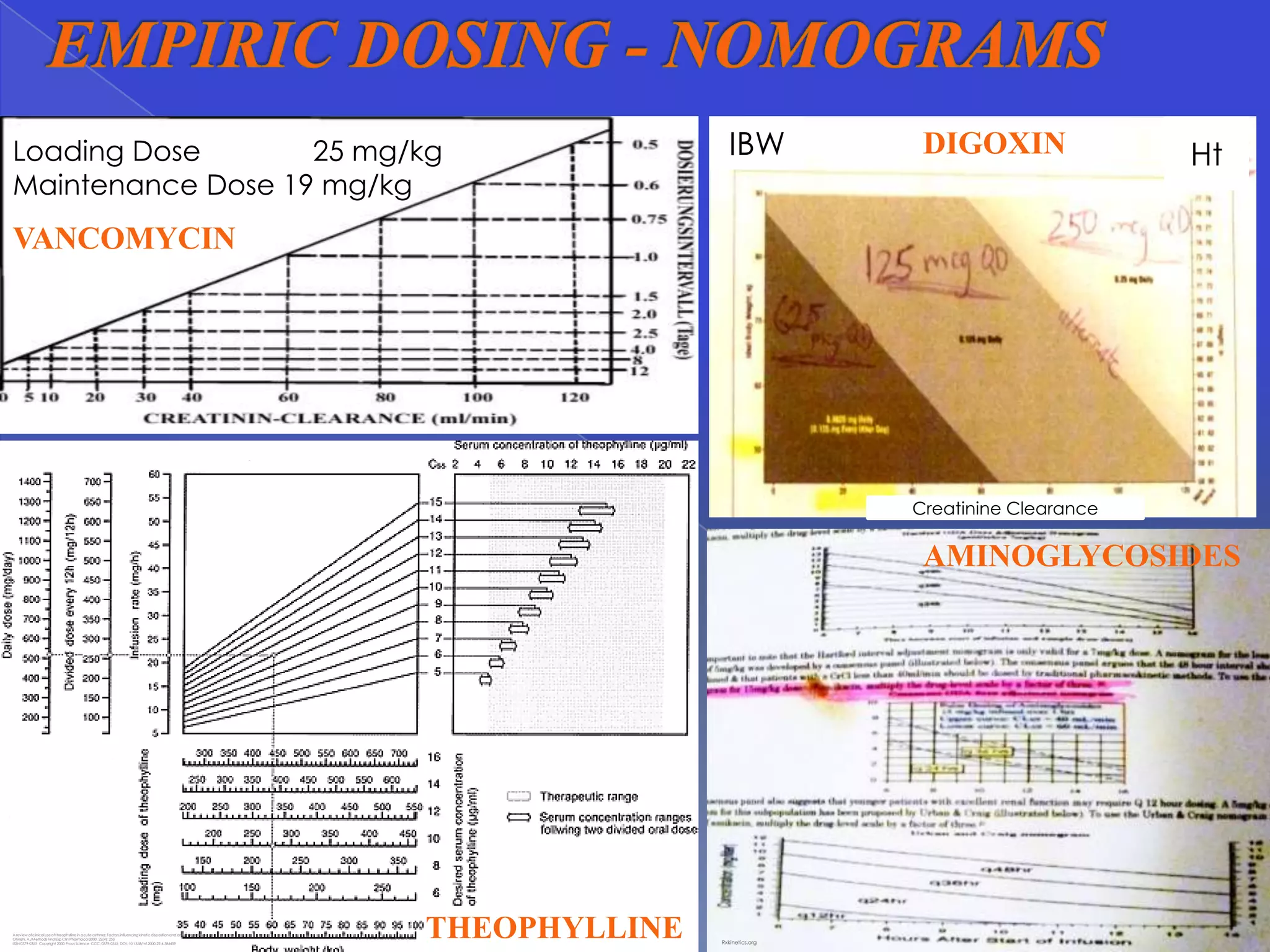
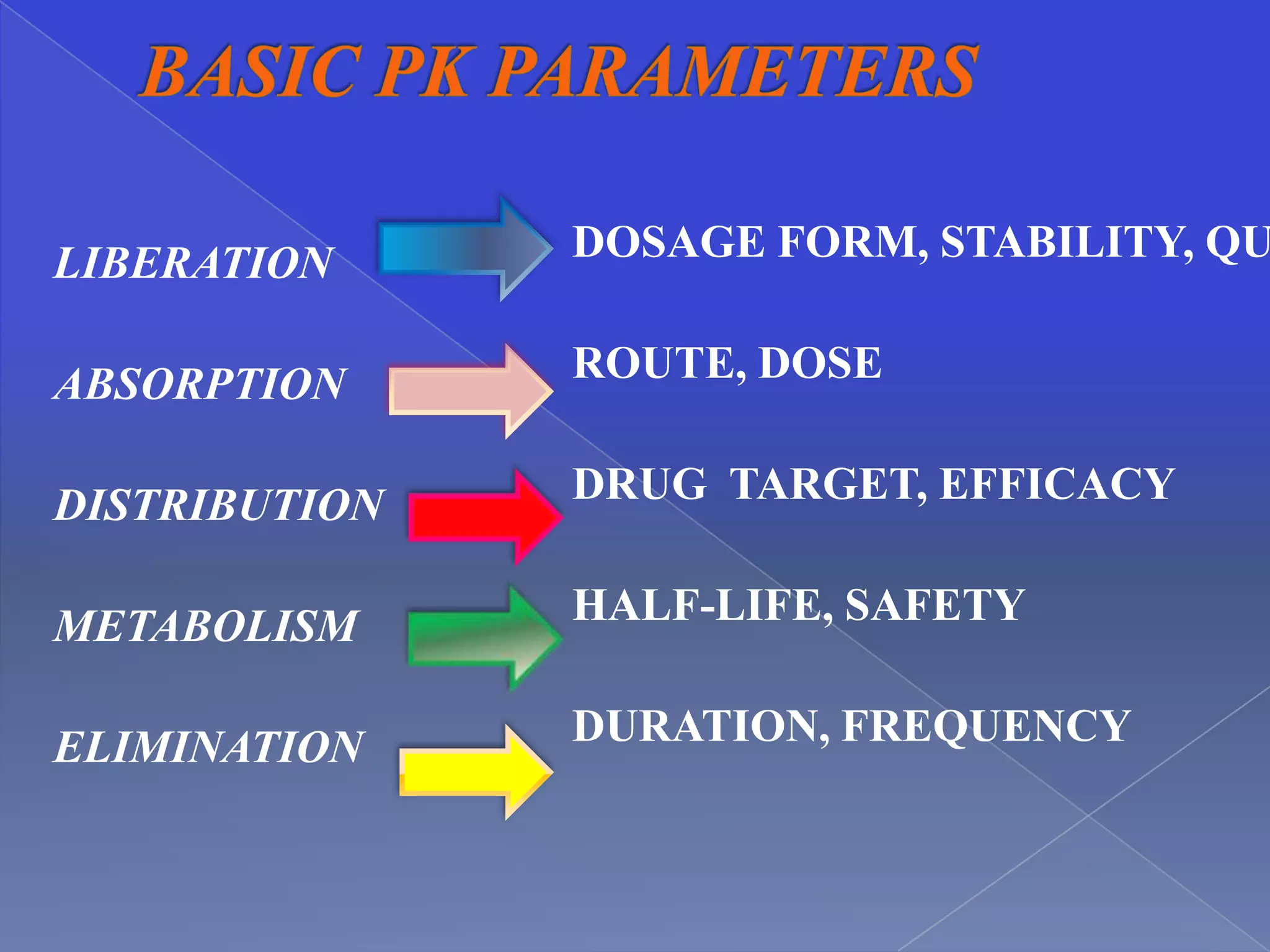
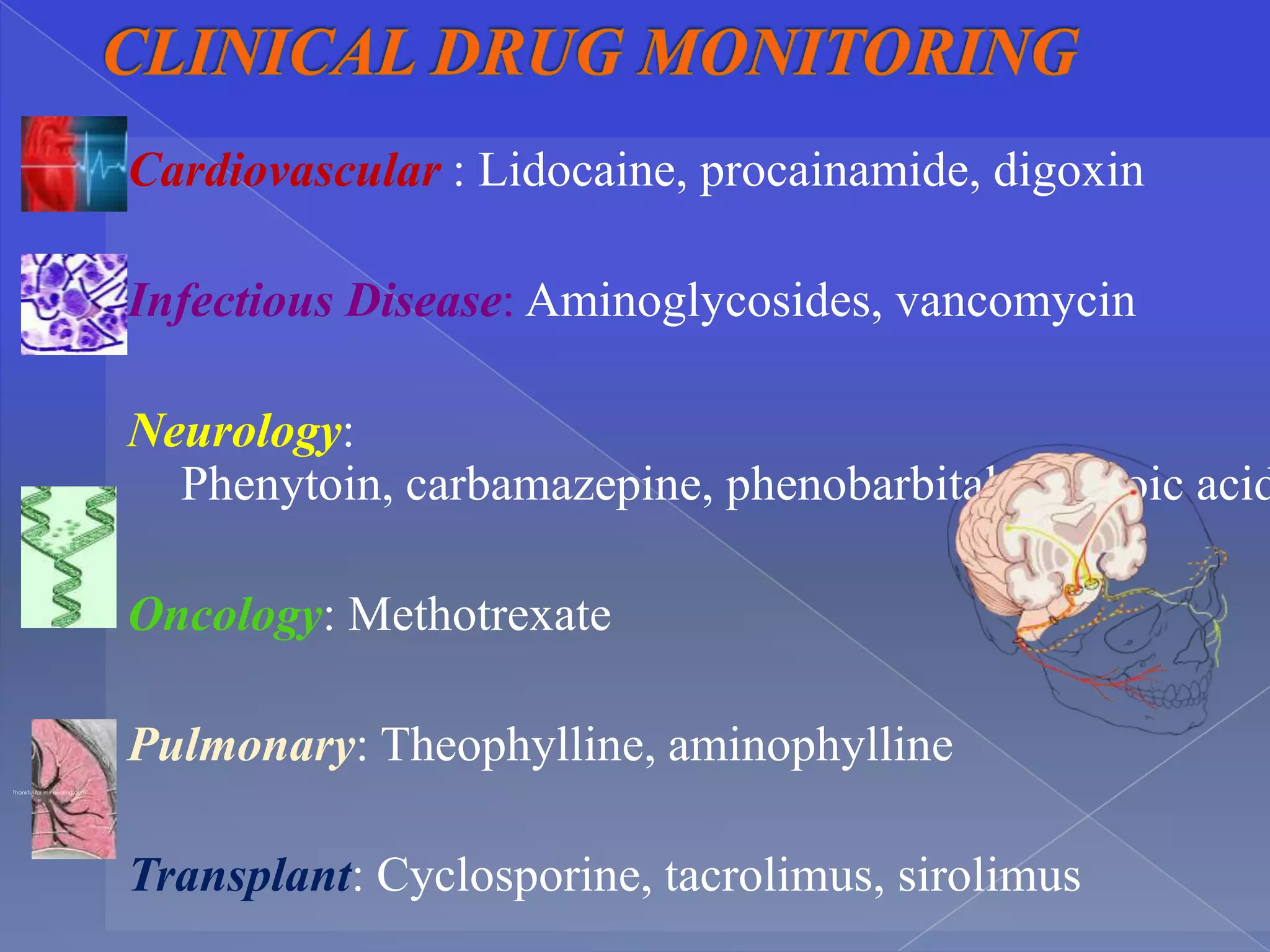
This document discusses key principles of pharmacokinetics including drug absorption, distribution, metabolism, and elimination. It provides examples of drugs that require therapeutic drug monitoring for several disease states, such as digoxin for cardiovascular issues, aminoglycosides for infectious diseases, and theophylline for pulmonary conditions. The document also outlines factors that influence pharmacokinetic parameters like body size, age, disease state, and drug interactions. Equations for calculating pharmacokinetic measures like loading dose, maintenance dose, and drug clearance are presented.

![ Intravenous: X0 = C0/Vd
Single IV: Ct future = C0* e-ket
Ct past = C0 / e-ket
Multiple IV: Dose= (Cp *Vd)(1-e-nkeτ /1-e-keτ)e-ket
Short-term Infusion (0.5 h), Multiple Dosing:
› R0= CL*Cmax{1-e-keτ /1-e-keT} e-ket 1
› Cmin= Cmax*e-ke(τ -T)
Continuous Infusion (1 h):
› R0= CL*Cpss*[1-e-keτ]/1-e-keT 1 ] e-ket 1
› Cmin= Cpss*e-ke(τ -T)
› 2R0/(C1+C2) + 2Vd (C1-C2) )/(C1+C2) (t1-t2)
Loading Dose: LD = Vd*Css / SF
Intermittent IV Bolus: Dose/τ = CL*Cp{1-e-keτ/1-e-keT}*e-ke(τ -T)
Maintenance Dose: MD =CL*Css* τ / SF
Oral:
› Multidose: Cave steady state = [LD/Vd*(1-e-keτ)] Ansel. Dosage Forms.
› Dose = C*(ka-ke)*Vd/SF*ka* (e-ket-e-kat)
Steady State: Multiple doses cause term 1-e-nket 1
australianprescriber.com](https://image.slidesharecdn.com/pharmacokineticspharmacodynamics-120408152856-phpapp01/75/Medicinal-Kinetics-Dynamics-4-2048.jpg)