The FDA guidance issued on December 20, 2017, outlines the classification pathways for medical device accessories, emphasizing that their classification is based on their associated risks rather than inherited from parent devices. Accessories must support, supplement, or augment the performance of a parent device, and the FDA has established a risk-based classification system following amendments to the FD&C Act. This guidance includes details on the regulatory processes for classifying new or existing accessories, including software as a medical device (SaMD), while clarifying that not all SaMD qualifies as an accessory.





![So what has changed?
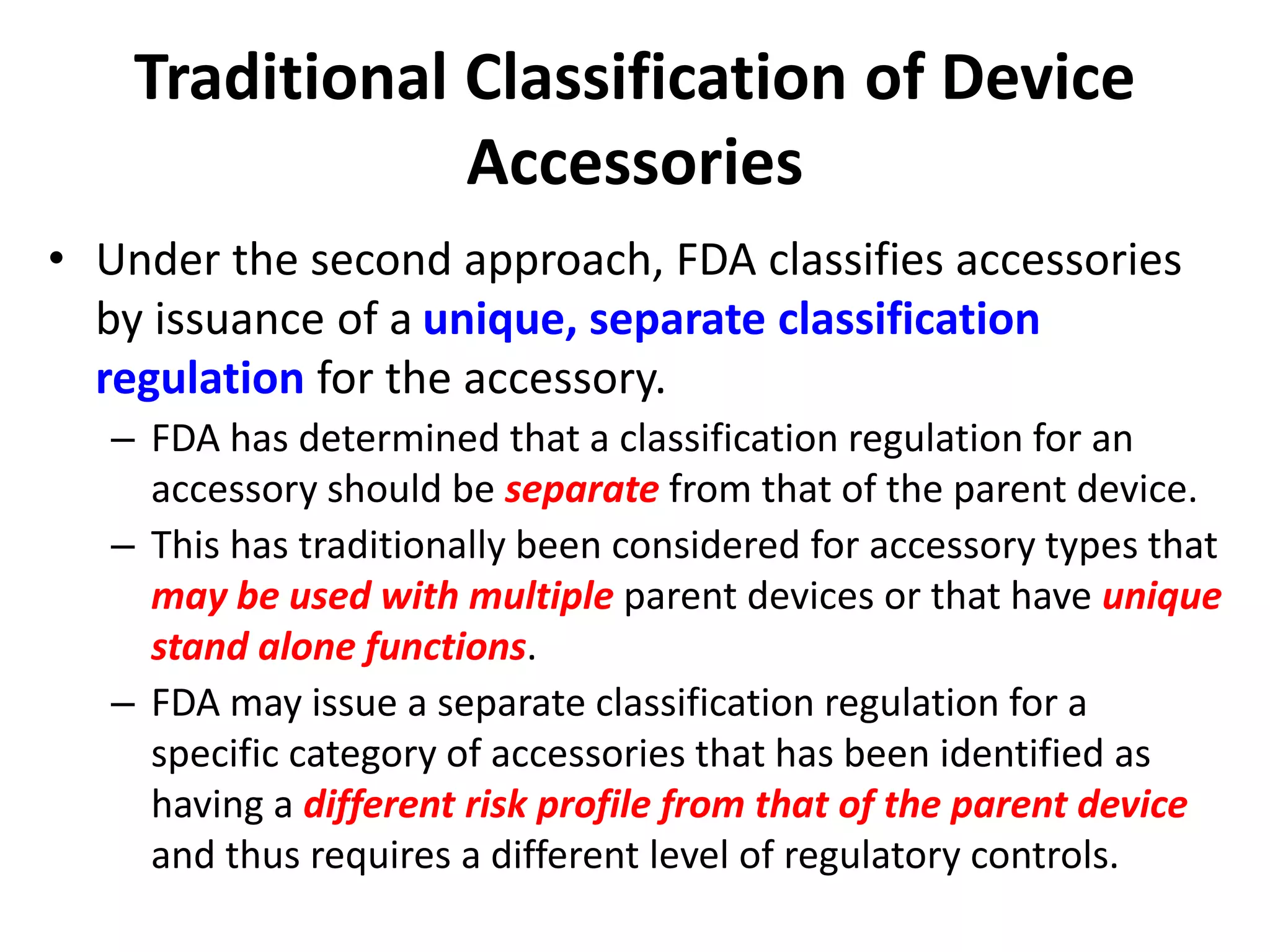
On August 18, 2017, section 513(f) of the FD&C Act was
amended by the FDA Reauthorization Act of 2017 (Public
Law 115-52) to state that
• “the Secretary shall … classify an accessory under
[section 513] based on the risks of the accessory when
used as intended and the level of regulatory controls
necessary to provide a reasonable assurance of safety
and effectiveness of the accessory, notwithstanding the
classification of any other device with which such
accessory is intended to be used.”](https://image.slidesharecdn.com/medicaldeviceaccessoriesdescribingaccessoriesandclassification-171224134251/75/Medical-device-accessories-describing-accessories-and-classification-7-2048.jpg)








![“In practice, the distinctions among devices that support, supplement, or
augment parent devices are subtle and many devices that meet the definition
of an accessory may do more than one of these things. Thus, if the device is
intended to support, supplement, and/or augment the performance of one or
more parent devices, [FDA] intend to consider the device to be an accessory
whether it is required or optional.”](https://image.slidesharecdn.com/medicaldeviceaccessoriesdescribingaccessoriesandclassification-171224134251/75/Medical-device-accessories-describing-accessories-and-classification-17-2048.jpg)








