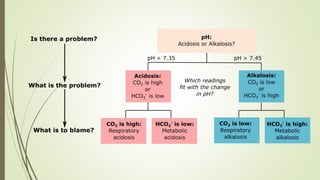
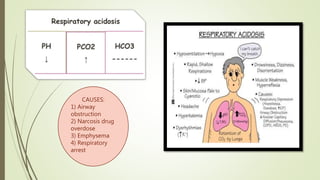
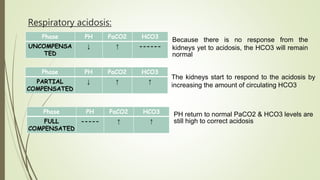
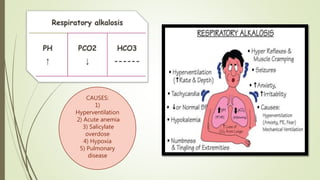
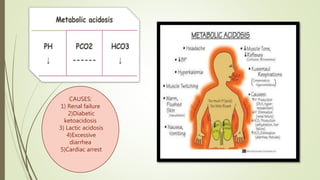
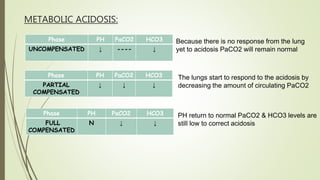
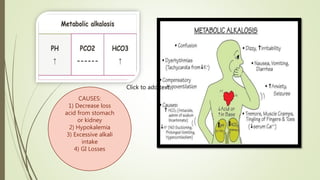
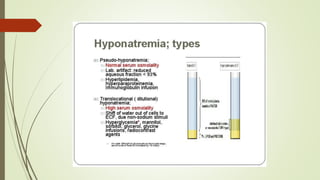
Mrs. A has signs and symptoms of respiratory distress including shortness of breath, cyanosis, tachycardia, and tachypnea. Her history of productive cough for 2 weeks suggests an underlying respiratory infection. An arterial blood gas would likely show respiratory acidosis with an elevated PCO2 and compensated or partially compensated bicarbonate, reflecting her body's attempt to counteract the respiratory acidosis through renal compensation.
















![CLINICAL MANIFESTATION
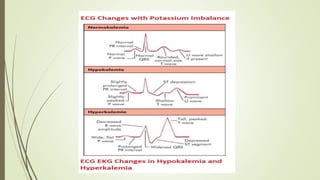
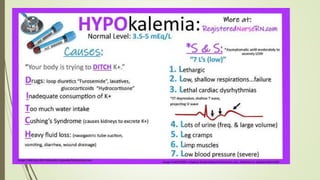
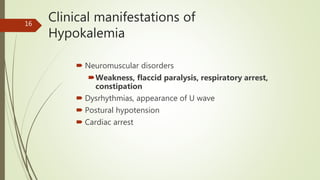
The most important effects of hyperkalemia are on skeletal and cardiac
muscle.
Skeletal muscle weakness is generally not seen until plasma [K +] is
greater than 8 mmol/L,
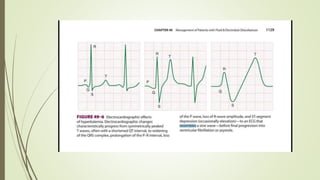
Cardiac manifestations are primarily due to delayed depolarization,
and are consistently present when plasma [K +] is greater than 7
mmol/L.](https://image.slidesharecdn.com/medicalcmefinal2-220727031836-b6815923/85/Medical-cme-final-2-pptx-23-320.jpg)
![TREATMENT
Hyperkalemia exceeding 6 mmol/L should always be corrected.
Triple regime (lytic cocktail)
-Iv calcium gluconate 10% 10cc 3-5 mins
-s/c actrapid 10U
-50cc 50% dextrose 30-60mins
Treatment is directed to reversal of cardiac manifestations and skeletal muscle weakness, and to
restoration of normal plasma [K+]
CONTINUOUS CARDIAC MONITORING, REPEAT RP in ½ hour
If persistent: HD
If <6.5: – Kalimate 5-10g TDS
– Resonium 15-30g TDS
– Neb 10g salbutamol – HTZ/loop diuretics](https://image.slidesharecdn.com/medicalcmefinal2-220727031836-b6815923/85/Medical-cme-final-2-pptx-25-320.jpg)