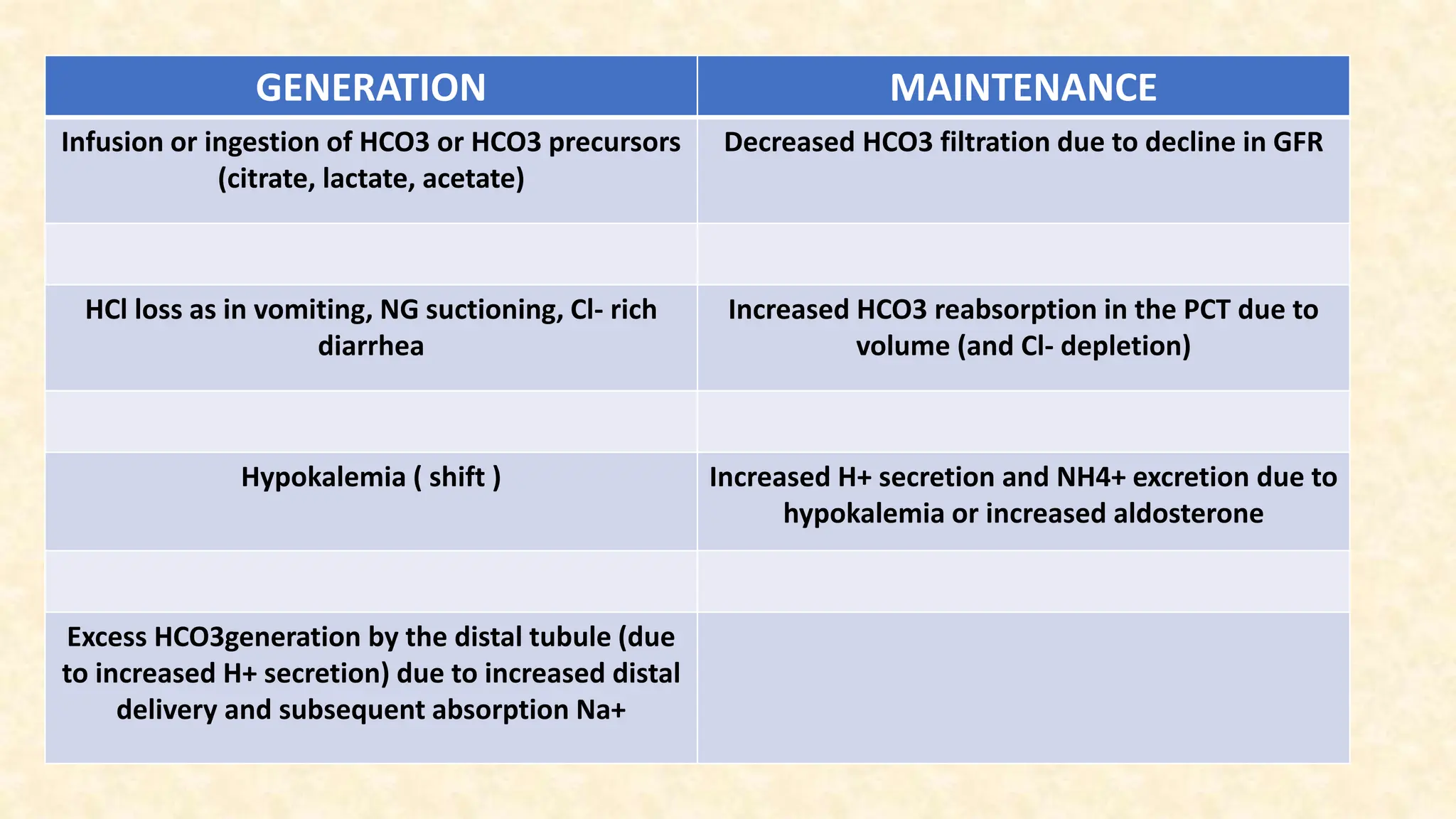
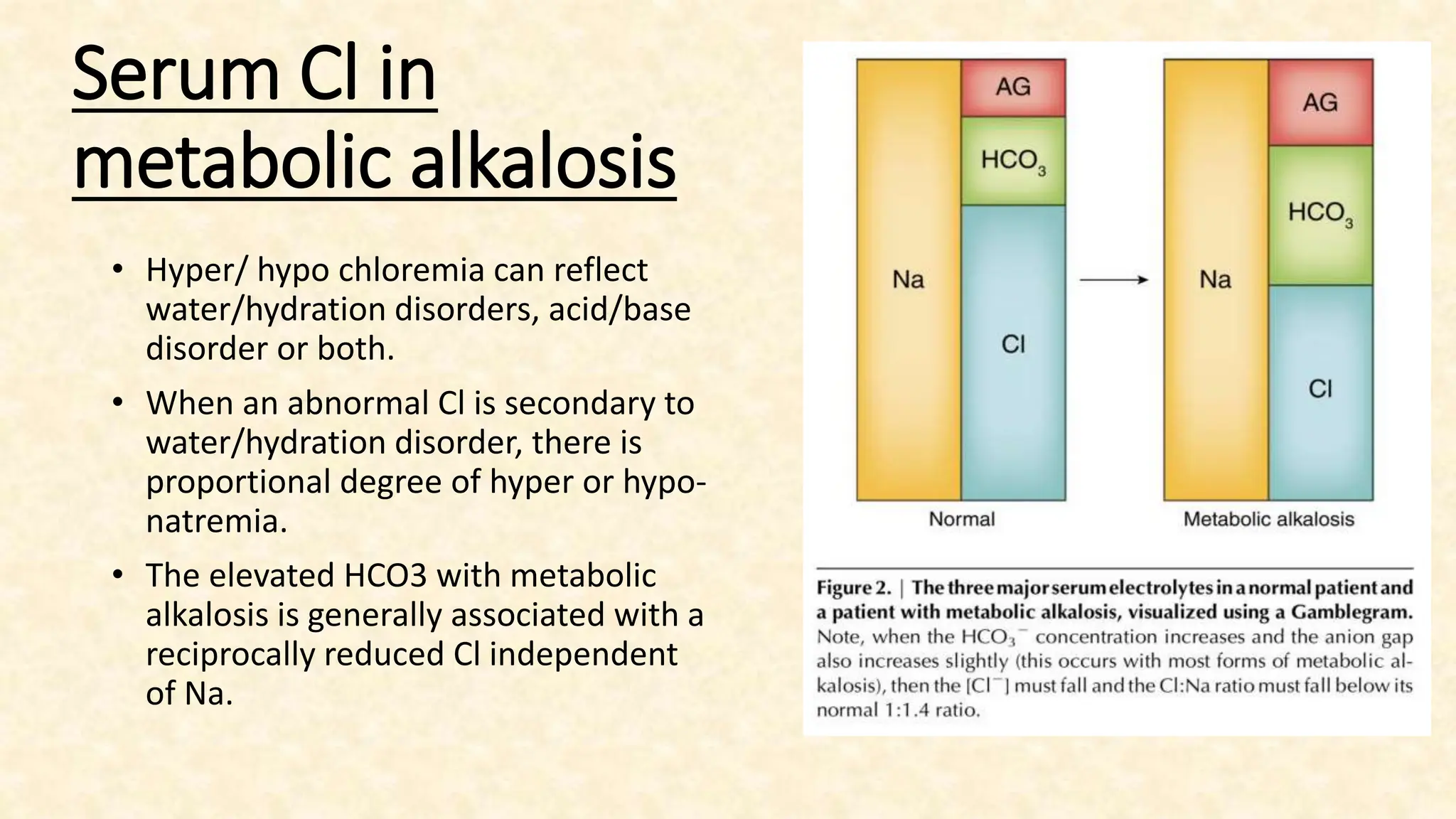
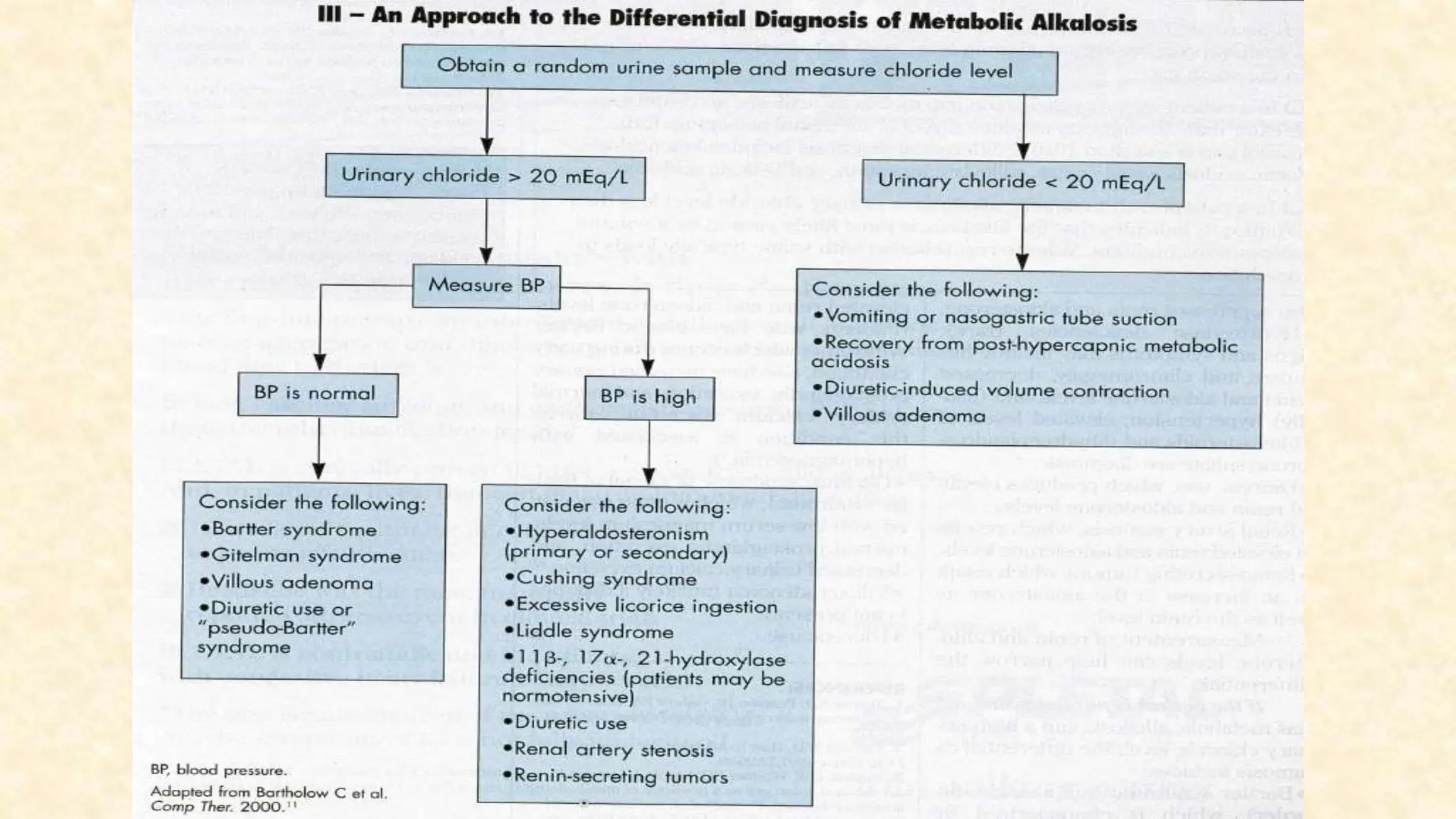
The document discusses metabolic alkalosis, outlining its definition, causes, and compensation mechanisms. It includes clinical case examples to illustrate the diagnosis and management of different acid-base disorders, alongside a discussion on respiratory conditions and their respective compensatory responses. Additionally, it poses several clinical questions related to acid-base balances for further analysis.