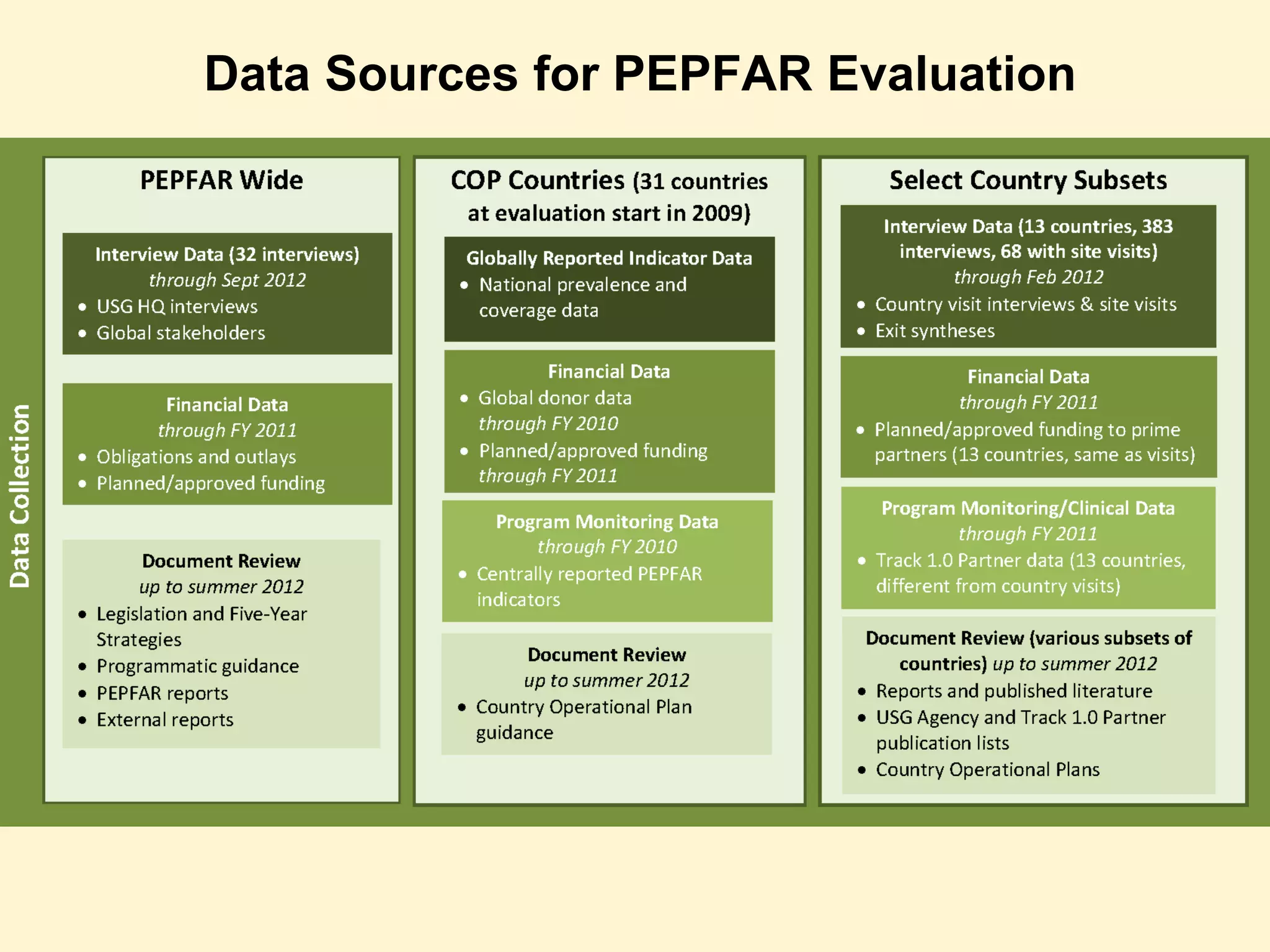
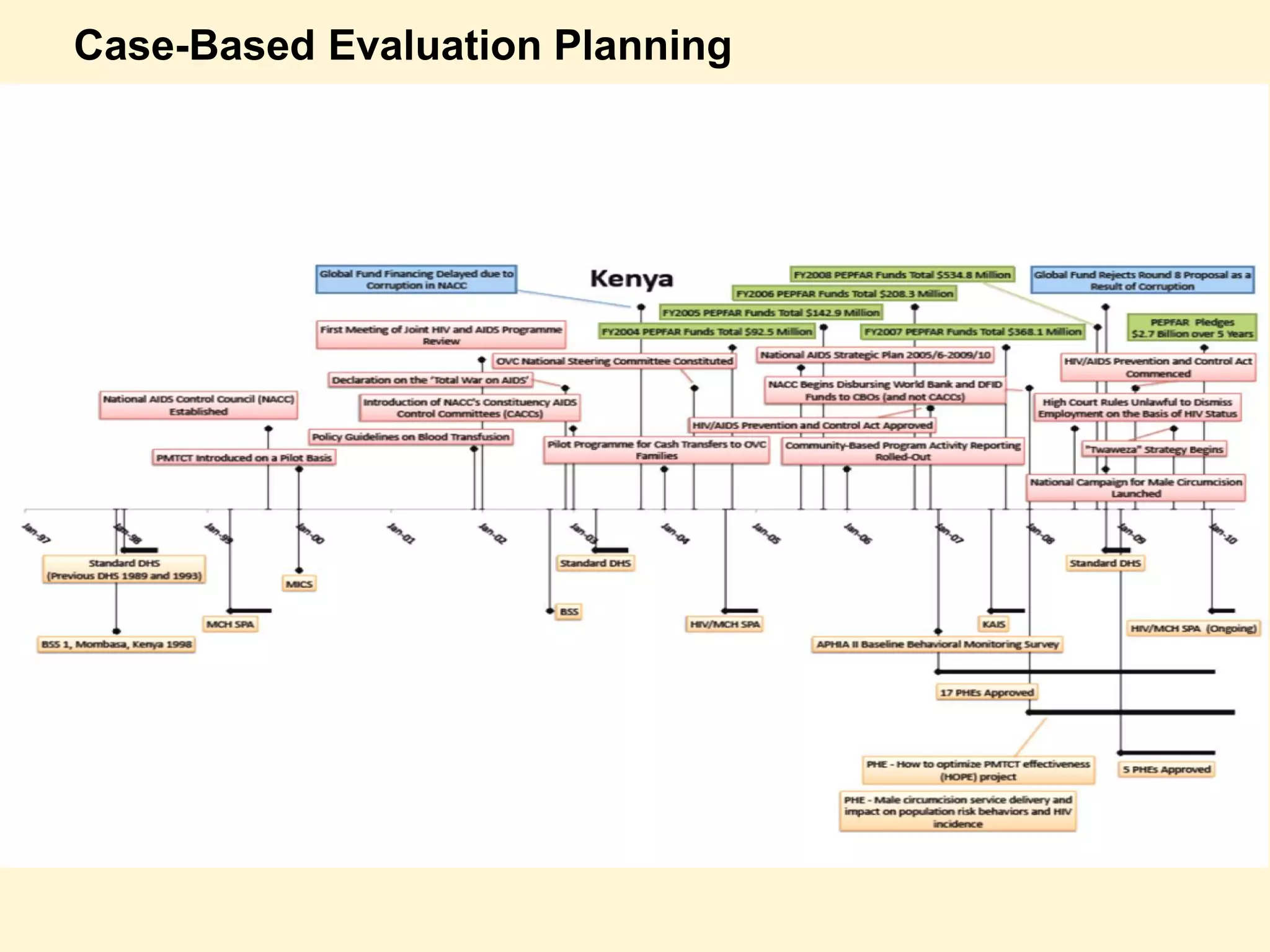
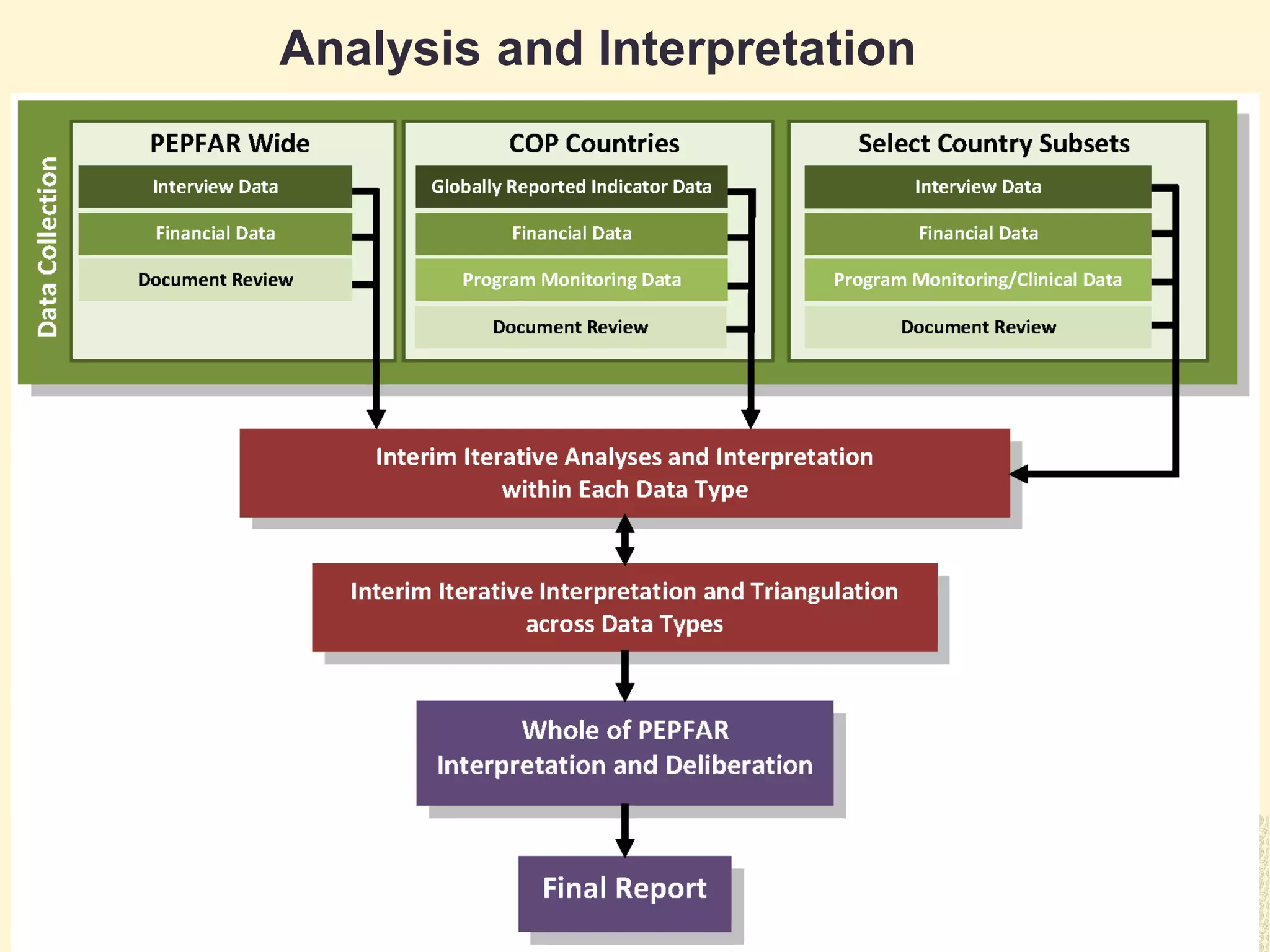
The document discusses the evaluation of PEPFAR conducted by the Institute of Medicine, focusing on the use of case-based approaches to assess its performance and health impact. It highlights the evaluation design, data sources, and methods used, emphasizing the importance of triangulation for thorough analysis. Key takeaways include the adaptability of case-based methods and the need for informed, intentional approaches in evaluation context.