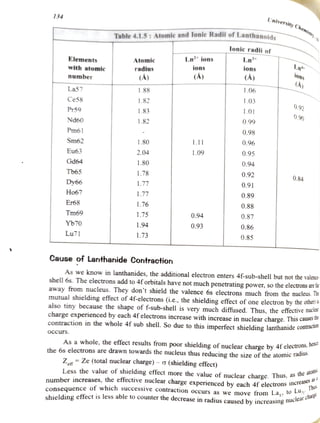
The document summarizes lanthanide contraction, which is the decrease in atomic and ionic radii as atomic number increases across the lanthanide series of elements. It occurs due to the imperfect shielding of the 4f electrons, which have low penetrating power and do not effectively shield the outer 6s electrons from the increasing nuclear charge. As atomic number increases, the effective nuclear charge felt by the 4f electrons increases, pulling the electrons closer to the nucleus and causing successive contraction across the series from lanthanum to lutetium. A table of atomic and ionic radii is provided to illustrate this steady decrease with increasing atomic number.