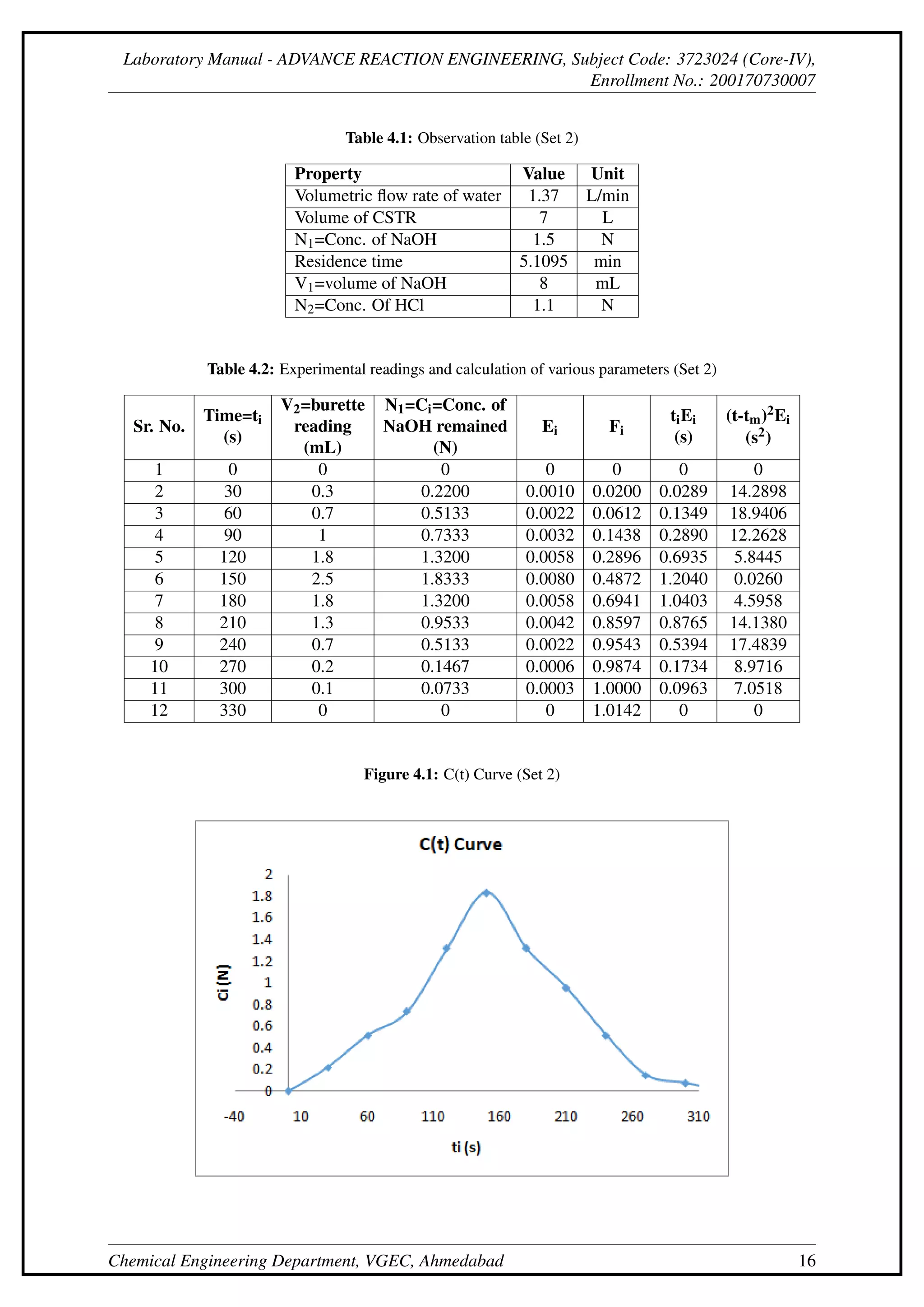
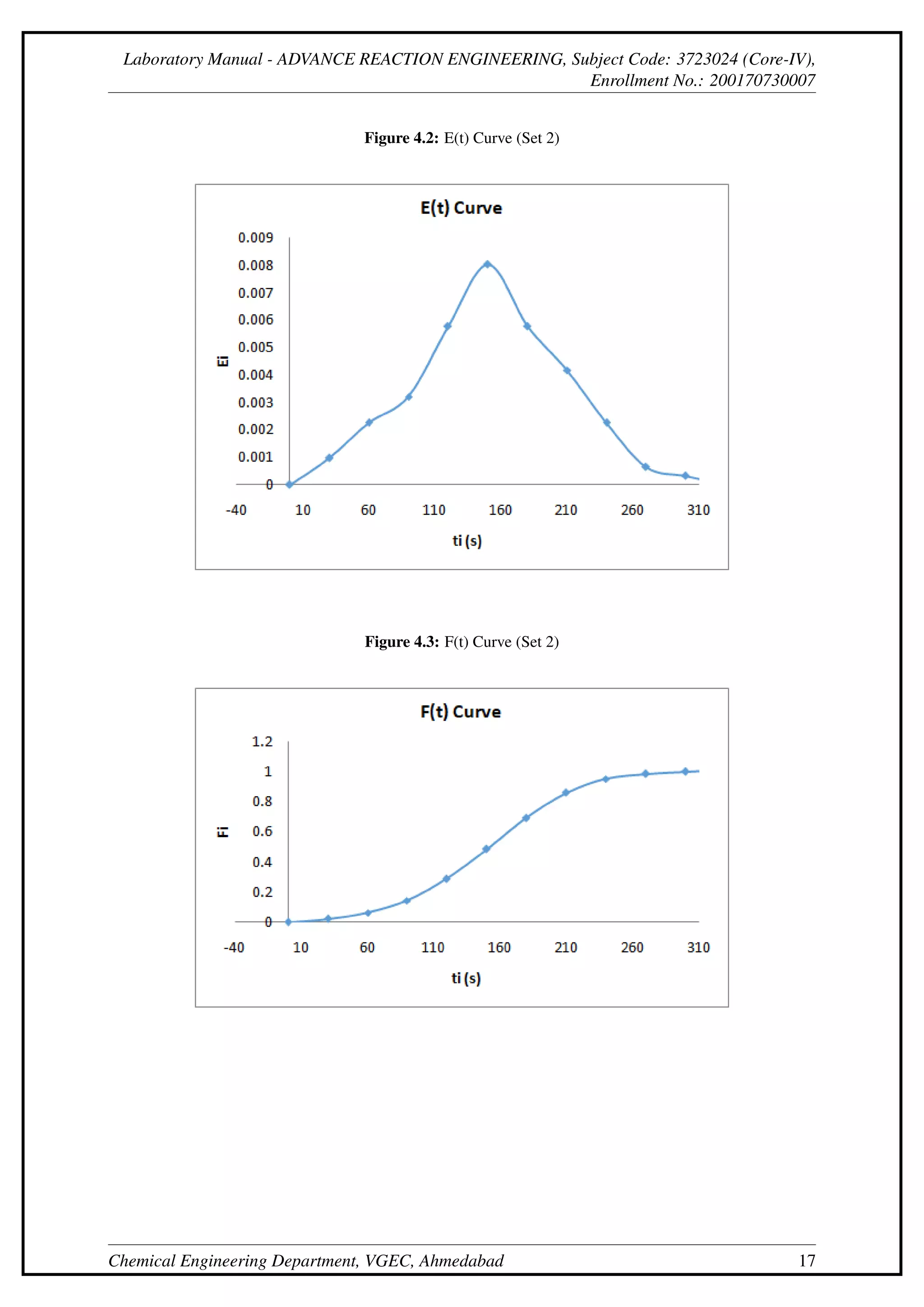
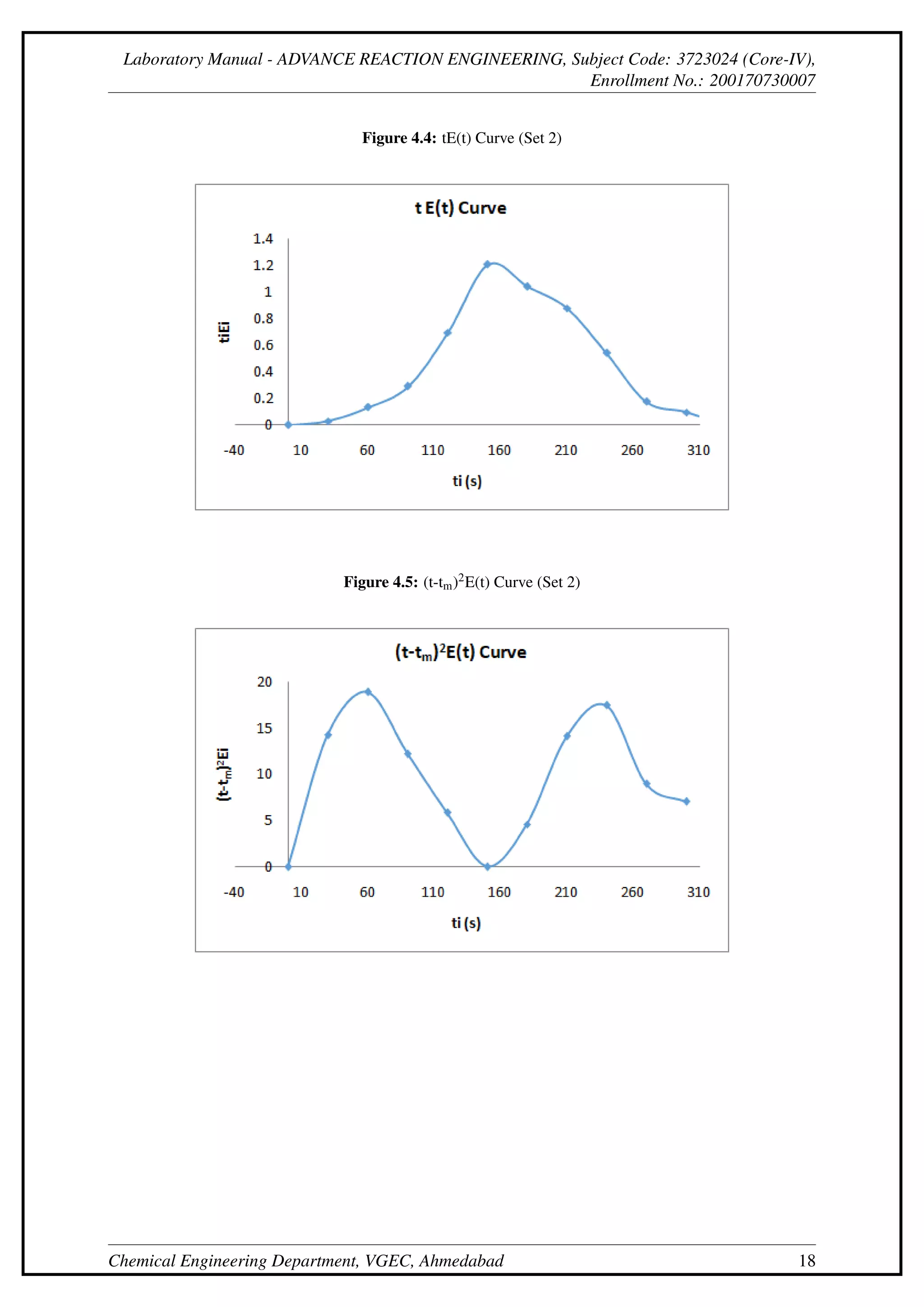
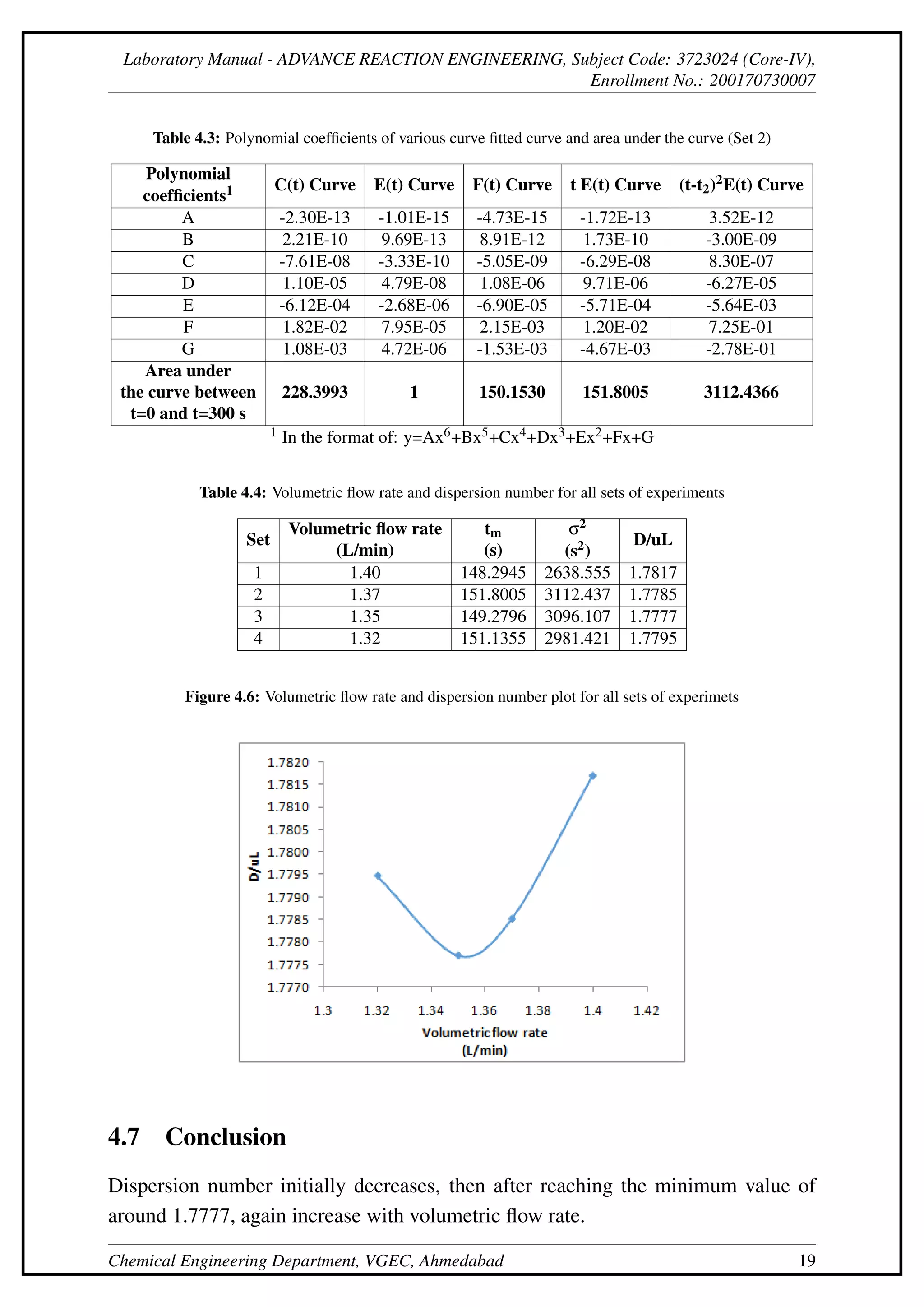
This document describes an experiment to determine the residence time distribution for a continuous stirred-tank reactor (CSTR) at different flow rates. Sodium hydroxide is injected as a tracer and samples are collected and titrated over time. Curves are constructed for concentration C(t), normalized concentration E(t), cumulative distribution F(t), and others. The dispersion number D/uL is calculated from these curves and found to initially decrease then increase with flow rate.