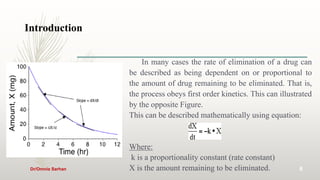
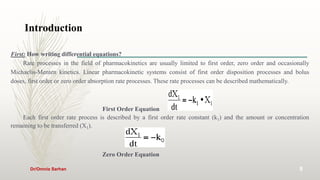
This document discusses pharmacokinetics and provides information about key concepts used in pharmacokinetics including:
- Logarithms and how they relate to calculating drug concentrations over time
- Differential and integral calculus which are used to develop equations to model rates of change in drug absorption and elimination over small time intervals
- How to write differential equations to model different rate processes like zero-order, first-order, and Michaelis-Menten kinetics
- The use of graphs including Cartesian and semi-log scales to plot drug concentration vs. time profiles
- Examples of using these concepts to solve pharmacokinetic problems