This document provides information on kidney transplantation and organ preservation techniques. It discusses:
- The shortage of donor organs compared to demand for transplantation. Donors can be living or deceased.
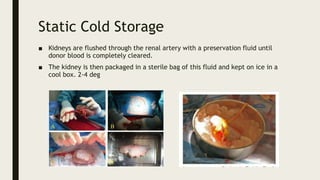
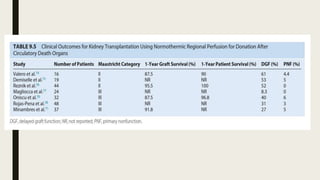
- Methods for preserving organs including static cold storage, hypothermic machine perfusion, normothermic regional perfusion, and normothermic machine perfusion. Preservation aims to suppress metabolism and minimize injury during storage.
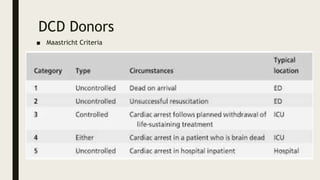
- Factors that can damage donor organs including brain death, circulatory death, warm ischemia, and reperfusion injury upon transplantation.
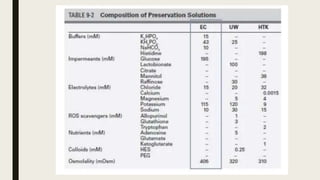
- Commonly used preservation solutions like University of Wisconsin, HTK, and Celsior and their mechanisms of action.
- Guidelines for maximum cold ischemia times