- InvenStar* CRC is a clinical research organization founded in 2008 by Dr. Shiva Murthy to support new drug development and clinical research according to ethical standards.
- The organization aims to provide quality clinical research services and has contracted over 5 projects with national and international clients in its first years of operation.
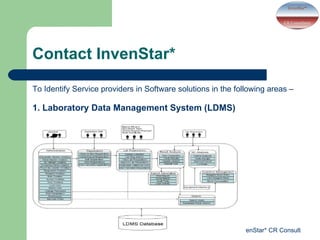
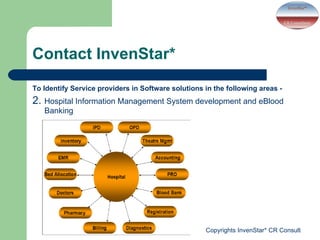
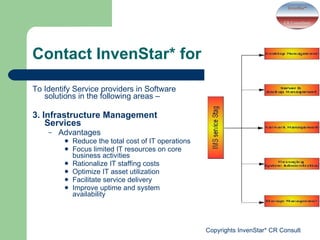
- InvenStar* CRC offers services including clinical trial support, medical education courses, and identifying software solution providers for applications like laboratory data management, hospital information systems, and eBlood banking. Revenue is also used for charitable activities through a trust.








![Thank You!!! For More Details Please Contact: Dr. Shiva Murthy. N, M.D. Chief Consultant [email_address] [email_address]](https://image.slidesharecdn.com/invenstarcrccapabilities-124525448776-phpapp01/85/InvenStar-CRC-Capabilities-16-320.jpg)