Embed presentation
Downloaded 26 times

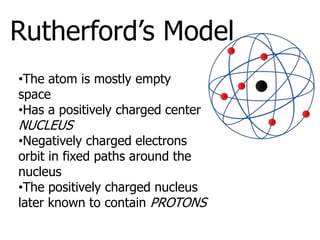
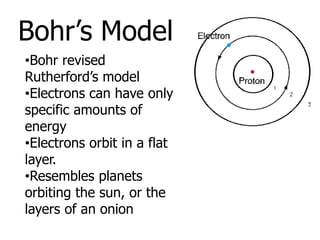
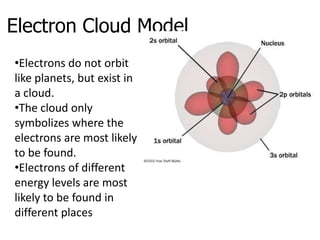
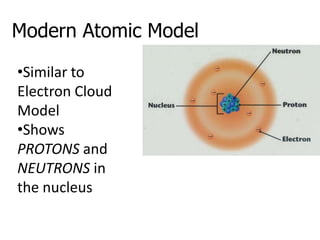
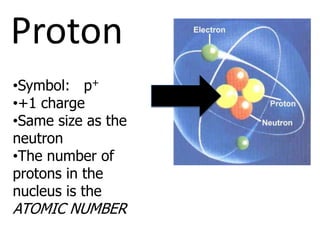
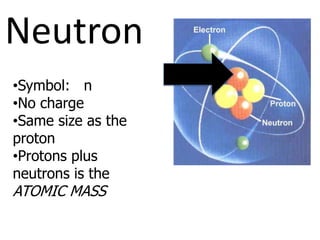
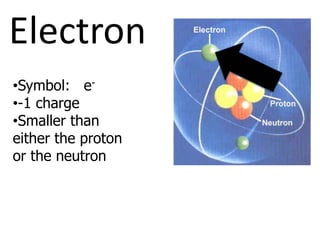
The document introduces several historical models of the atom: Thomson's "plum pudding" model, Rutherford's nuclear model with electrons orbiting a dense nucleus, Bohr's model incorporating allowed electron energy levels, and the modern electron cloud model showing the probabilistic distribution of electrons rather than definite orbits. It notes key characteristics of protons, neutrons, and electrons - their relative charge and size, and roles in determining atomic number and mass.








