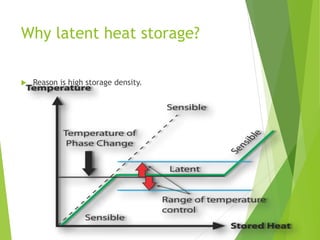
This document discusses phase change materials (PCMs) for energy storage applications. It begins with an introduction to PCMs and their characteristics like high energy storage density and ability to store/release energy at a constant temperature. It then covers classifications of organic and inorganic PCMs and compares their properties. Challenges like low thermal conductivity of organic PCMs are discussed along with solutions like adding thermal conductivity enhancers. The document concludes that PCMs are promising for energy storage due to their high storage density and ability to store/release energy at constant temperatures.











![solution
Varying the composition of PCM by incorporating other
materials like embedded aluminum powder into paraffin
wax reduced the charging time by 60%[1] .
Embedding Xgnp [graphite nano particles] up to 7% into
paraffin increased, thermal conductivity from 0.2 to
0.8W/MK](https://image.slidesharecdn.com/pcmsecondppt-181018150238/85/introduction-to-pcm-13-320.jpg)
![continued
Next approach to solve thermal conductivity problem is
to vary the design of storage tank.
So for shell and tube type design is most analyzed.
Its established by various research works the most
suitable configuration is to have heat transfer fluid
within the tube and PCM around it within the shell [2,3]](https://image.slidesharecdn.com/pcmsecondppt-181018150238/85/introduction-to-pcm-14-320.jpg)


![References
[1] A. Sharma et al.: Review on thermal energy storage
with phase change materials and applications.
Renewable and Sustainable Energy Reviews,
vol.13, 2009, pp. 318-345.
[2] S. Kim, L.T. Drzal: High latent heat storage and high
thermal conductive phase change materials
using exfoliated graphite nano platelets. Solar
Energy Materials & Solar Cells, vol. 93,2009,
pp. 136-142.
[3] Thomas Hasenöhrl: An Introduction to Phase Change
Materials as Heat Storage Mediums Project
Report 2009 MVK 160 Heat and Mass Transport
May 09, 2009, Lund, Sweden.](https://image.slidesharecdn.com/pcmsecondppt-181018150238/85/introduction-to-pcm-17-320.jpg)