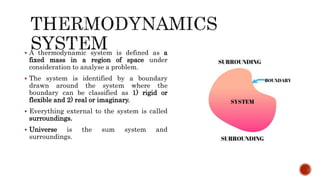
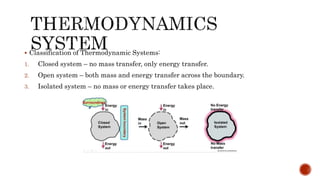
The document provides an overview of thermodynamics, defining it as the science of energy transfers and their effects on systems, and outlines its four laws. It discusses the characteristics of thermodynamic systems, including closed, open, and isolated systems, along with the concepts of state and properties, which are classified into intensive and extensive types. Additionally, the document explains thermodynamic equilibrium and quasi-static processes, emphasizing the conditions required for equilibrium and the nature of reversible processes.