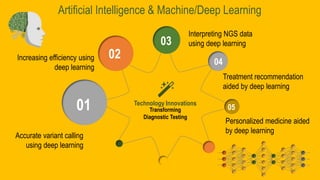
The Genomic Testing Cooperative (GTC), established by Dr. Maher Albitar, aims to integrate AI with next-generation sequencing to provide affordable genomic profiling for cancer across communities. The cooperative model allows shared resources and economies of scale, enhancing cost-effectiveness and access to advanced technology and expertise in genomic testing. GTC offers comprehensive genomic profiling services, focusing on both solid and hematologic tumors, while providing options for both low and high-volume practice groups.