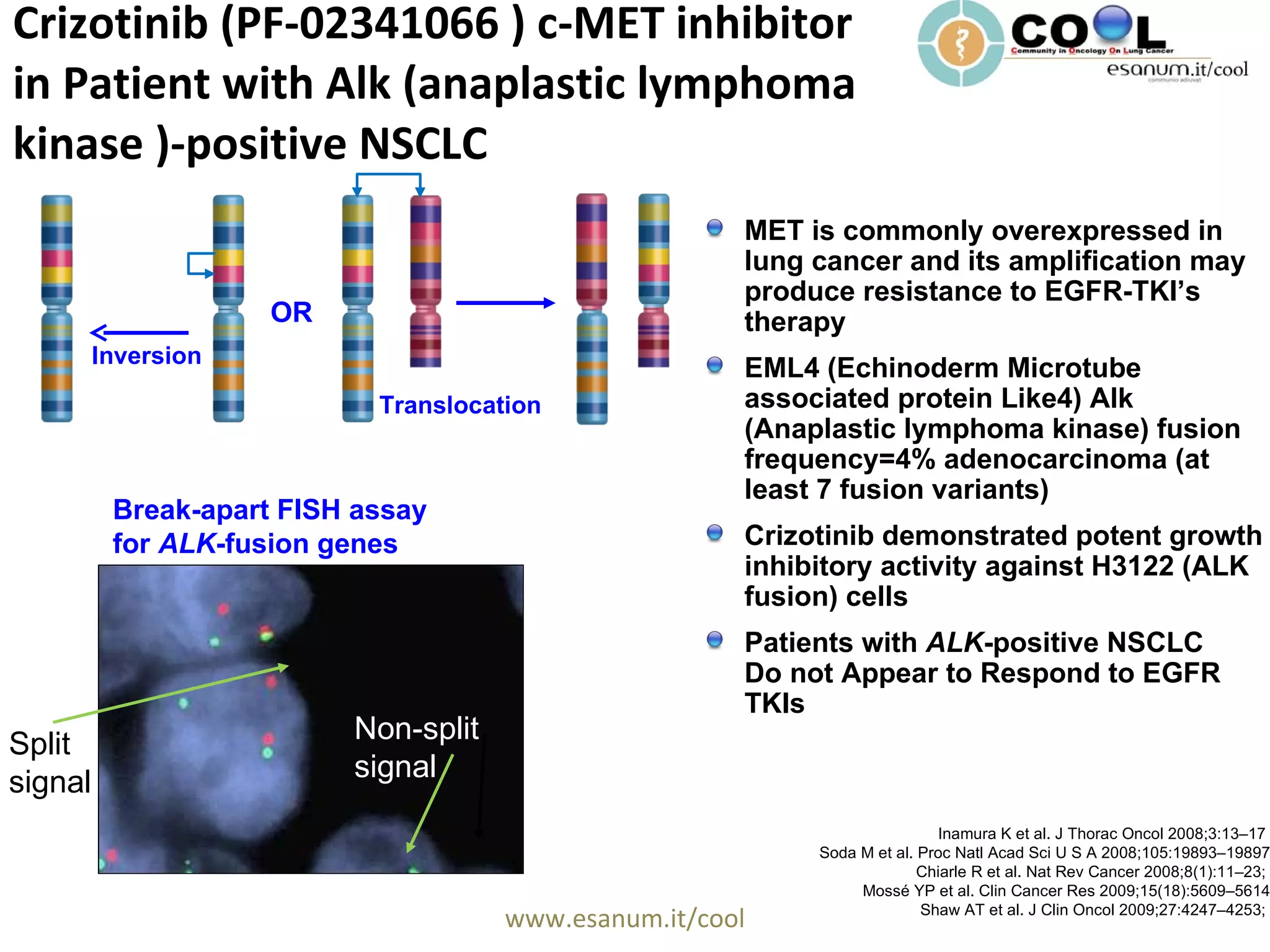
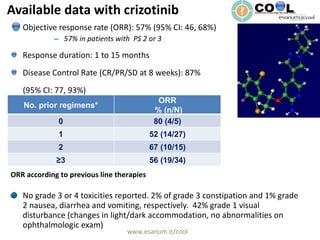
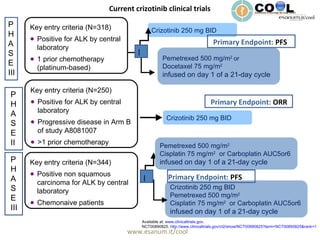
Crizotinib is a c-MET inhibitor that has demonstrated potent inhibitory activity against ALK fusion cells. The document discusses clinical trials of crizotinib in patients with ALK-positive non-small cell lung cancer (NSCLC). Results from Phase I and II trials showed an objective response rate of 57% and disease control rate of 87% with crizotinib. Current ongoing trials are evaluating crizotinib versus chemotherapy as first-line or second-line treatment in ALK-positive NSCLC.