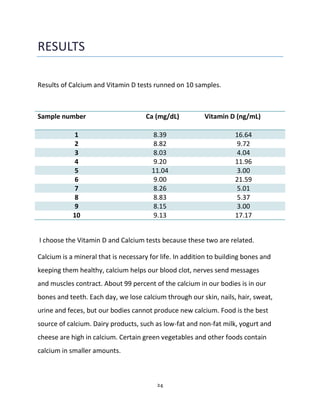
Sandra Aouf completed a medical internship from June 15th to July 20th, 2014 at the Specialized Medical Center Hospital in Riyadh, Saudi Arabia. During the internship, she was trained in the chemistry and special chemistry section of the clinical laboratory department. She learned about the various machines used, including the Cobas 6000 analyzer series which performs tests via photometry, ion-selective electrodes, and immunoassays. As part of her training, Sandra determined calcium, vitamin D, and HBsAg levels in patient samples using these different techniques.
















![17
Reaction:
Ca2+ + o-CPC → calcium-o-CPC complexe
The color intensity of the complex formed is directly proportional to the calcium
concentration and is measured photometrically.
The Reagents:
R1: CAPS (3-[cyclohehaxylamino]-1-propanesulfonic acid) 525 mmol/L ;
NaOH: 400 mmol/L , pH 11.5 ; nonreactive surfactant
R2: o-cresolphthalein complexone : 0.5 mmol/L ; 8-hydroxyquinoline : 30
mmnol/L ; pH 1.1 ; Stabilizer
Limitations-Interferance :
Icterus , Hemloysis,Lipemia,Drogues : no significant interference.
Reference range:
Serum/plasma
Children (0-10days): 7.6 – 10.4 mg/dL
Children (10 days- 2 years): 9.0 – 11.0 mg/dL
Children (2-12 years): 8.8 – 10.8 mg/dL
Children (12-18 years): 8.4 – 10.2 mg/dL
Adults (18-60 years): 8.6 – 10.0 mg/dL
Adults (60-90 years): 8.8 – 10.2 mg/dL
Adults (> 90 years): 8.2 – 9.6 mg/dL](https://image.slidesharecdn.com/5fae6ef1-359c-4d6c-acfa-45556e3f685c-160126213057/85/Internship-report-18-320.jpg)