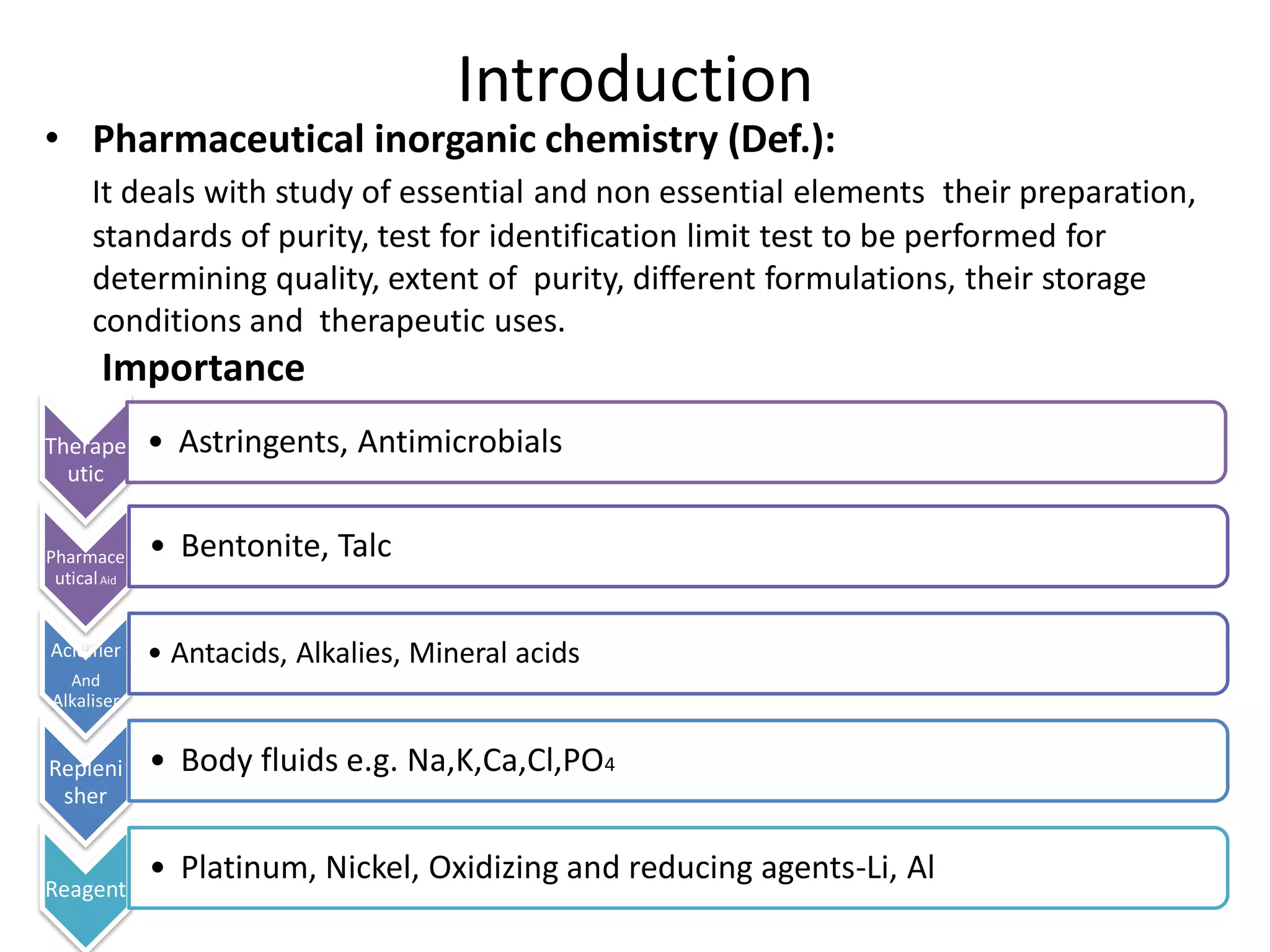
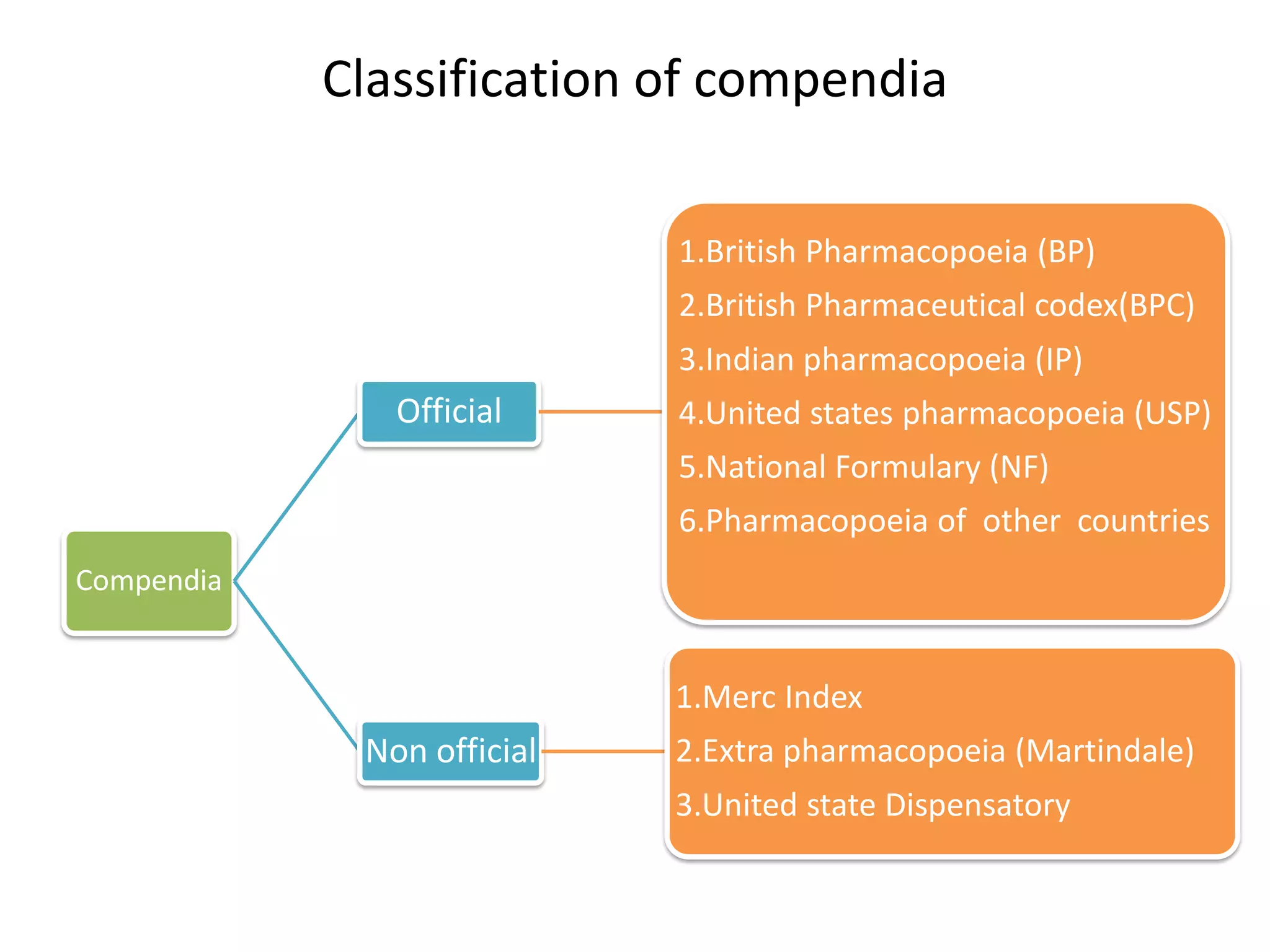
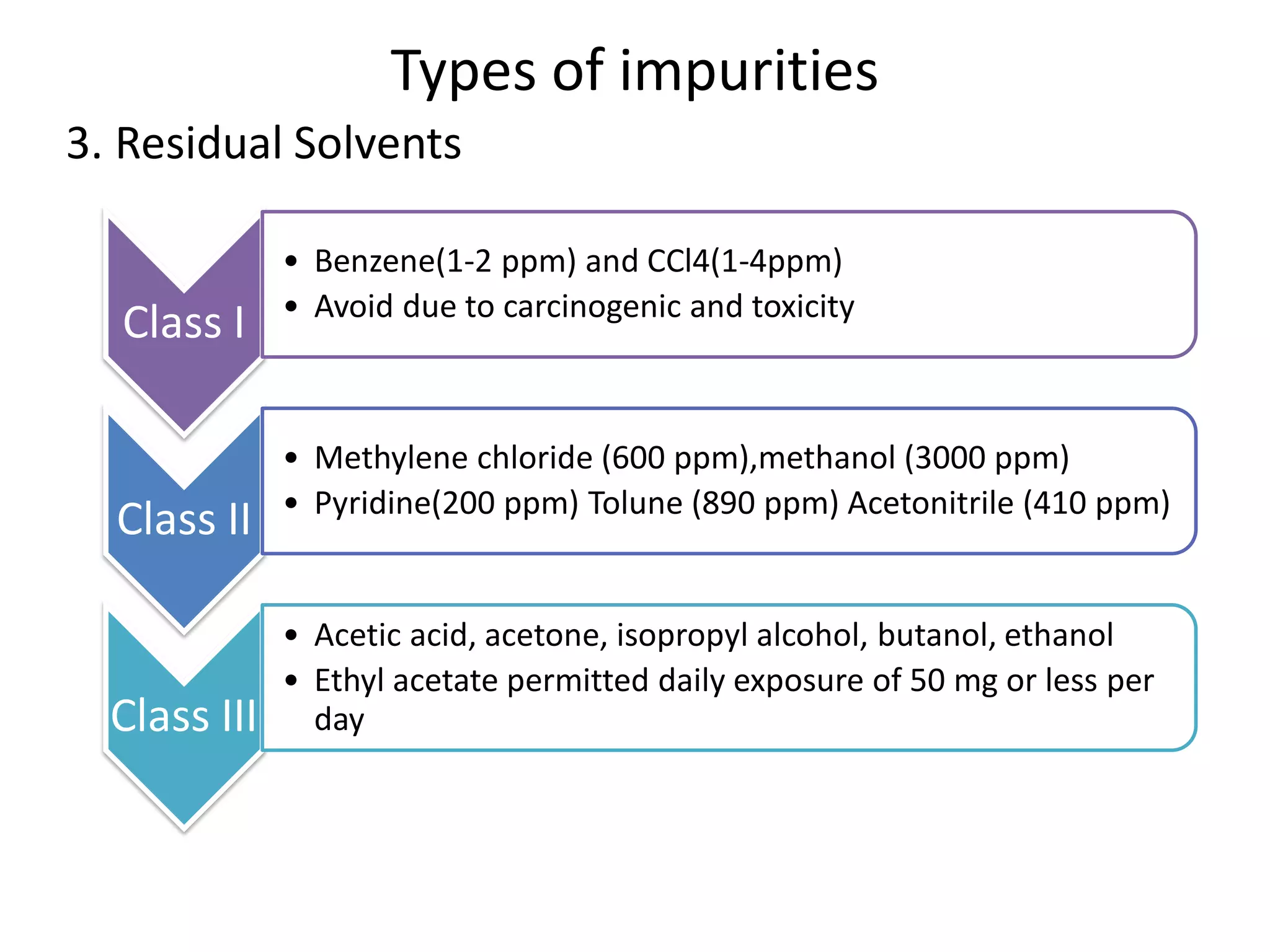
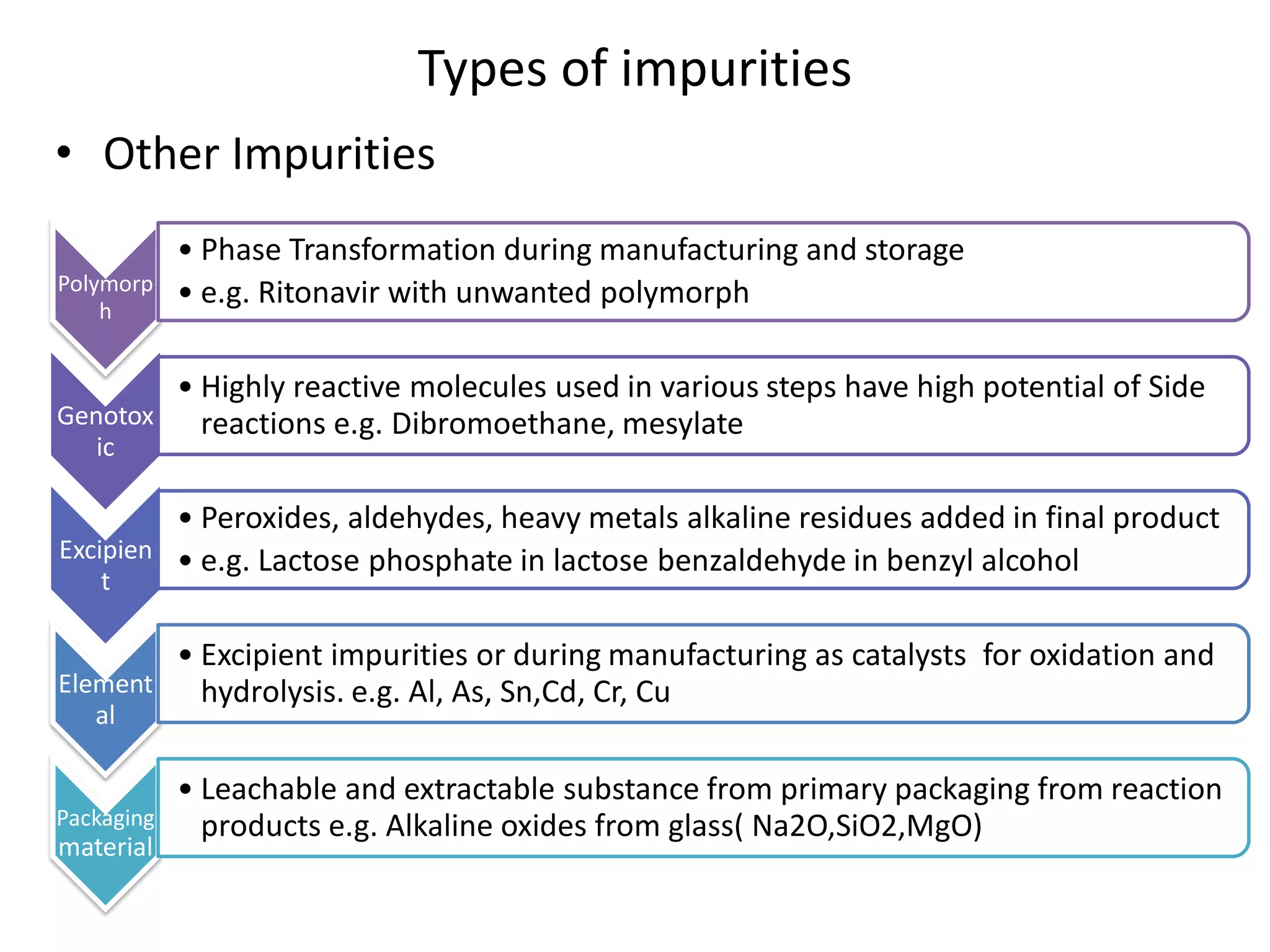
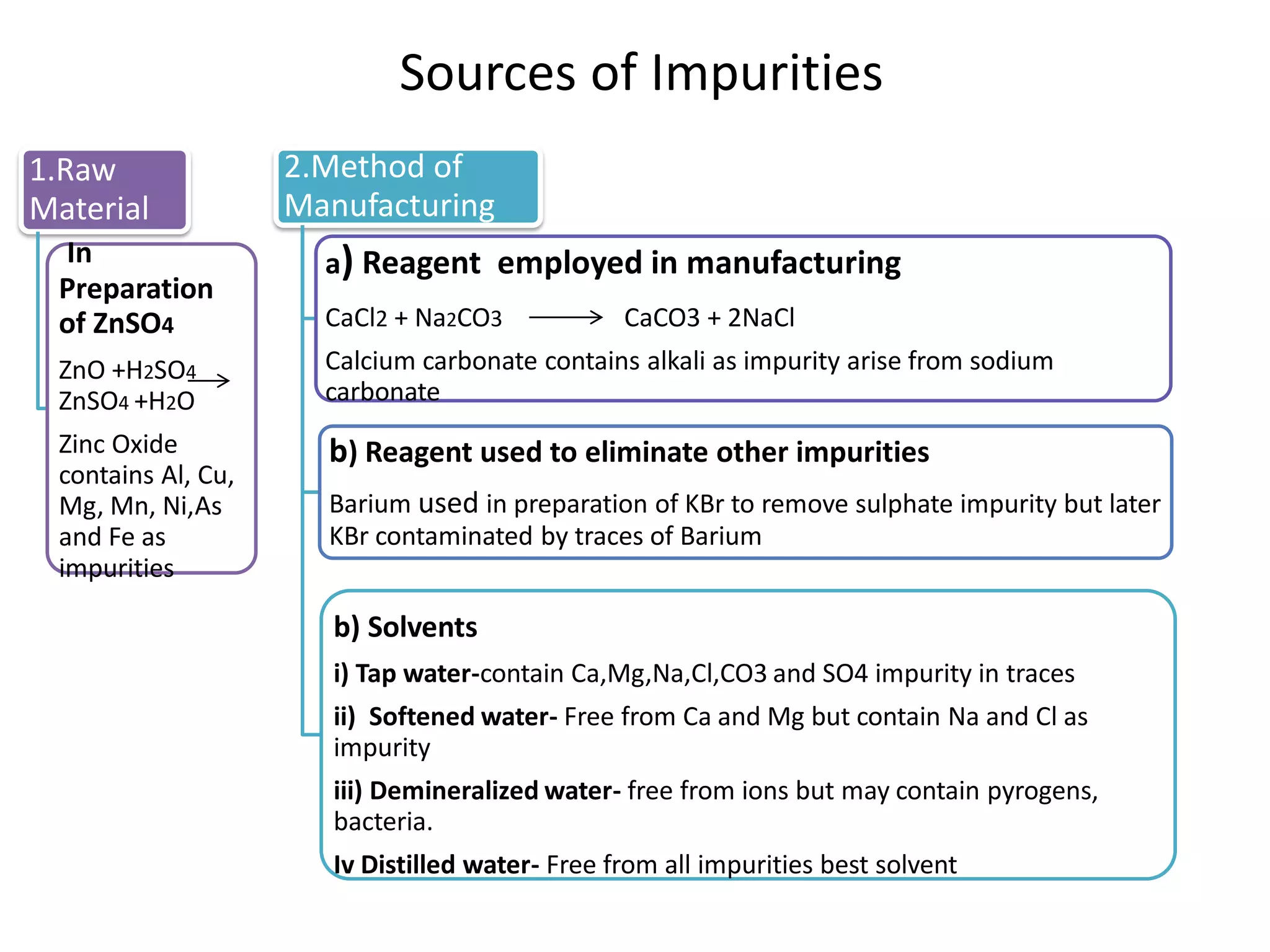
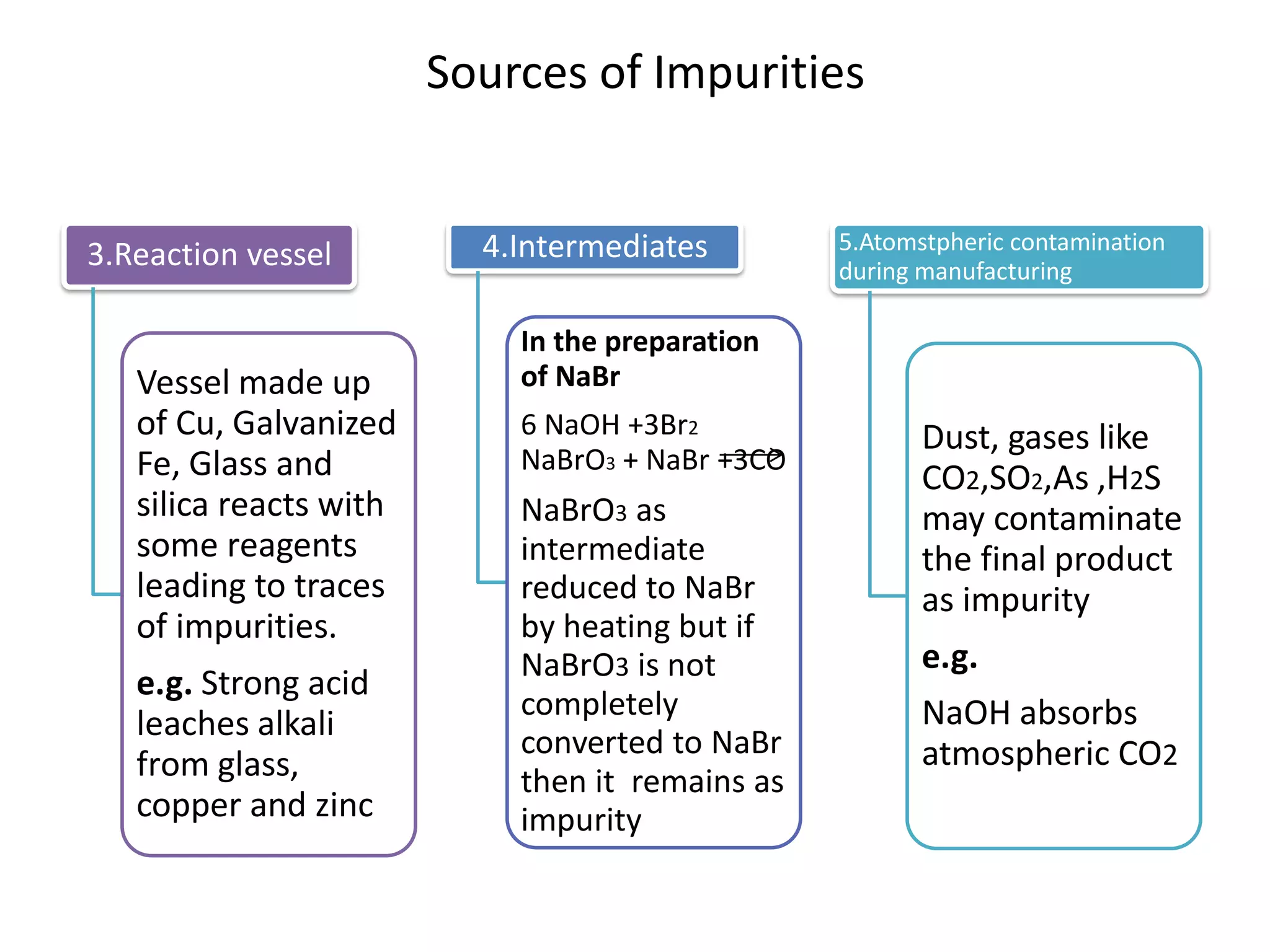
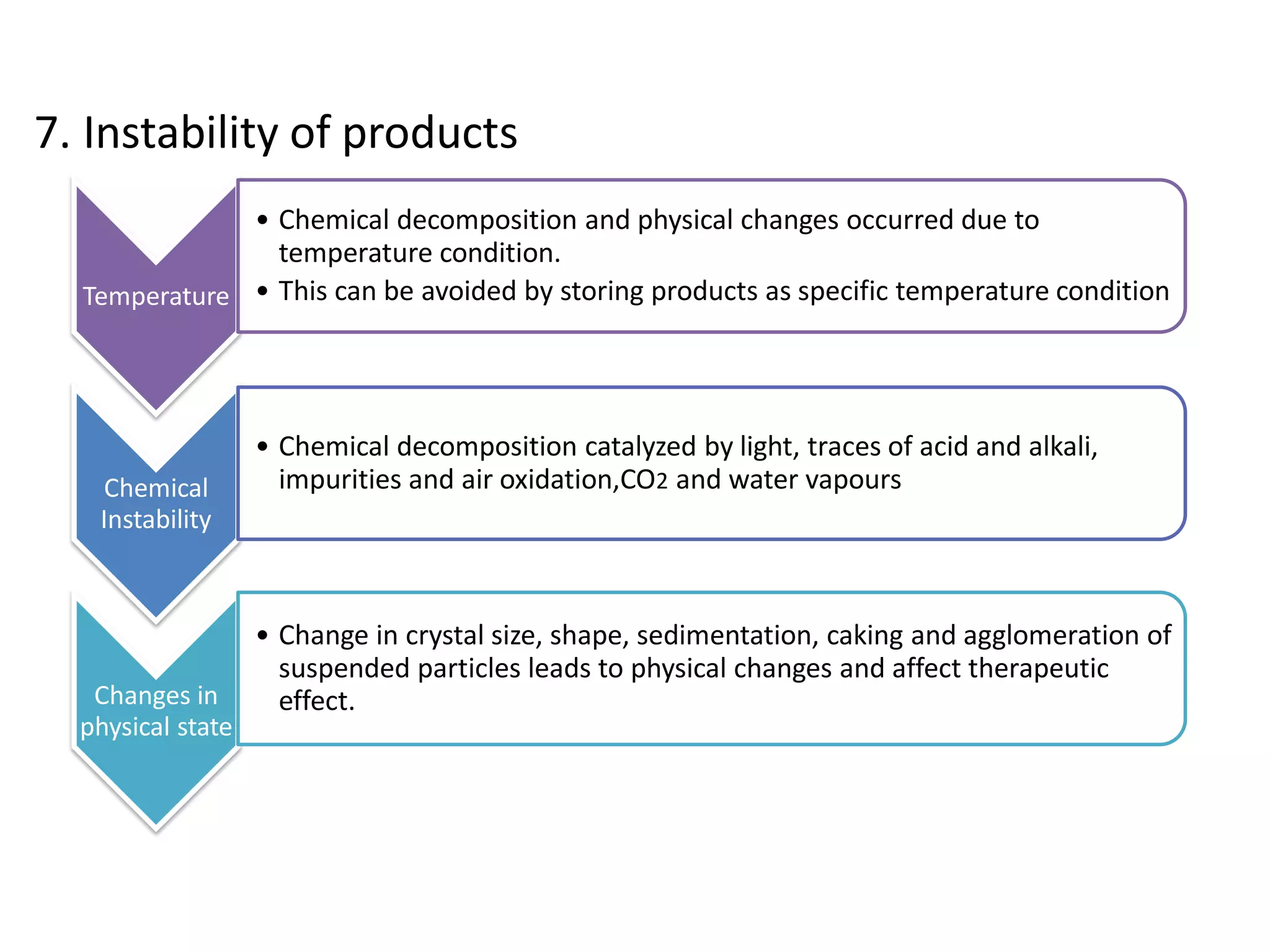
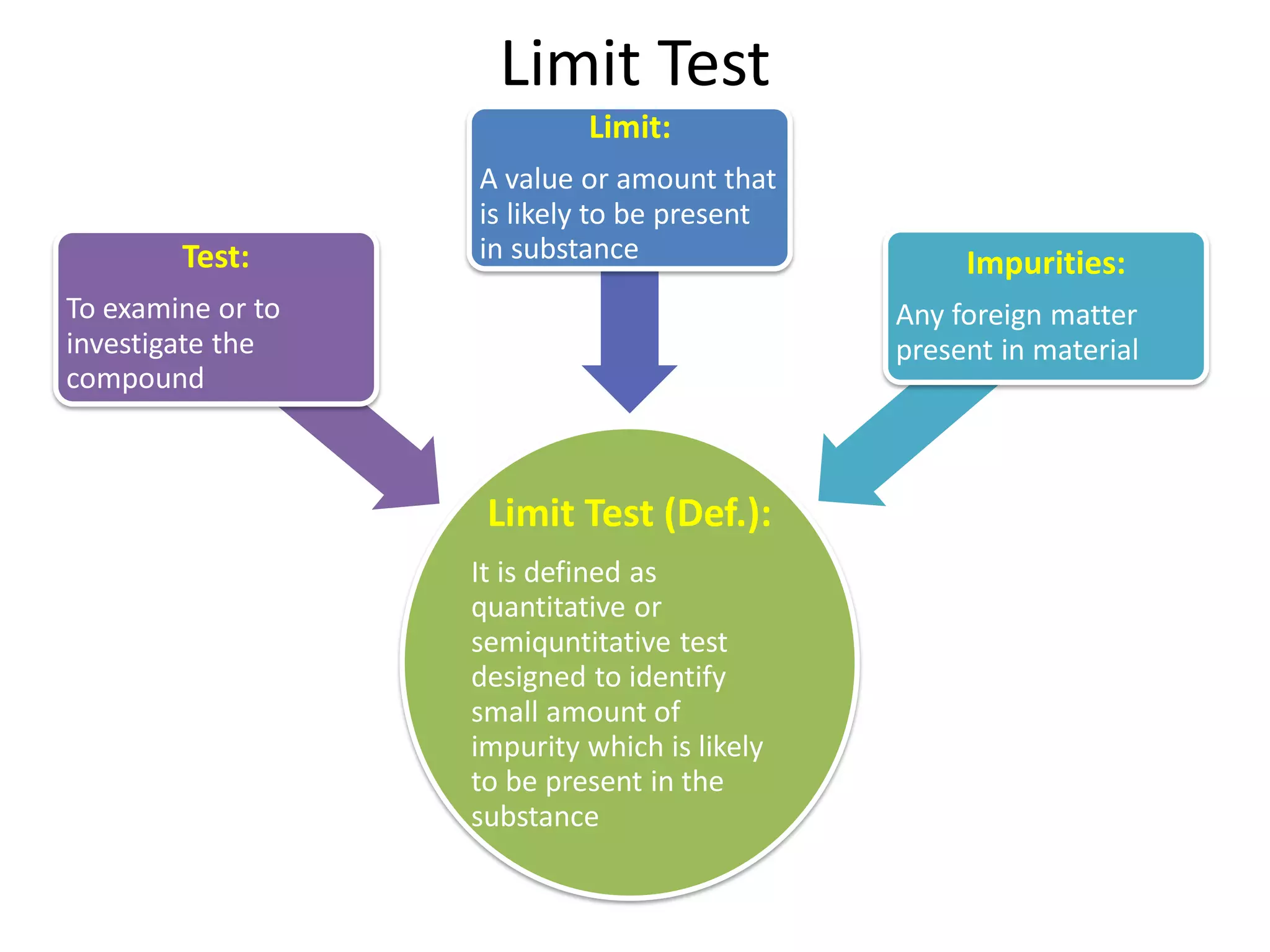
The document discusses various types of impurities that may be present in pharmaceutical substances and their sources. It classifies impurities as organic (such as byproducts, degradation products, intermediates, reagents), inorganic (heavy metals, residual solvents), and other types (polymorphs, genotoxic, excipients, packaging materials). The sources of impurities are described as the raw materials, the manufacturing methods and reagents used, and the reaction vessels. Contamination can occur at various stages from raw materials, solvents, reagents, filtration aids, air, water, and equipment. Strict quality control measures are needed to minimize impurities and ensure drug safety.