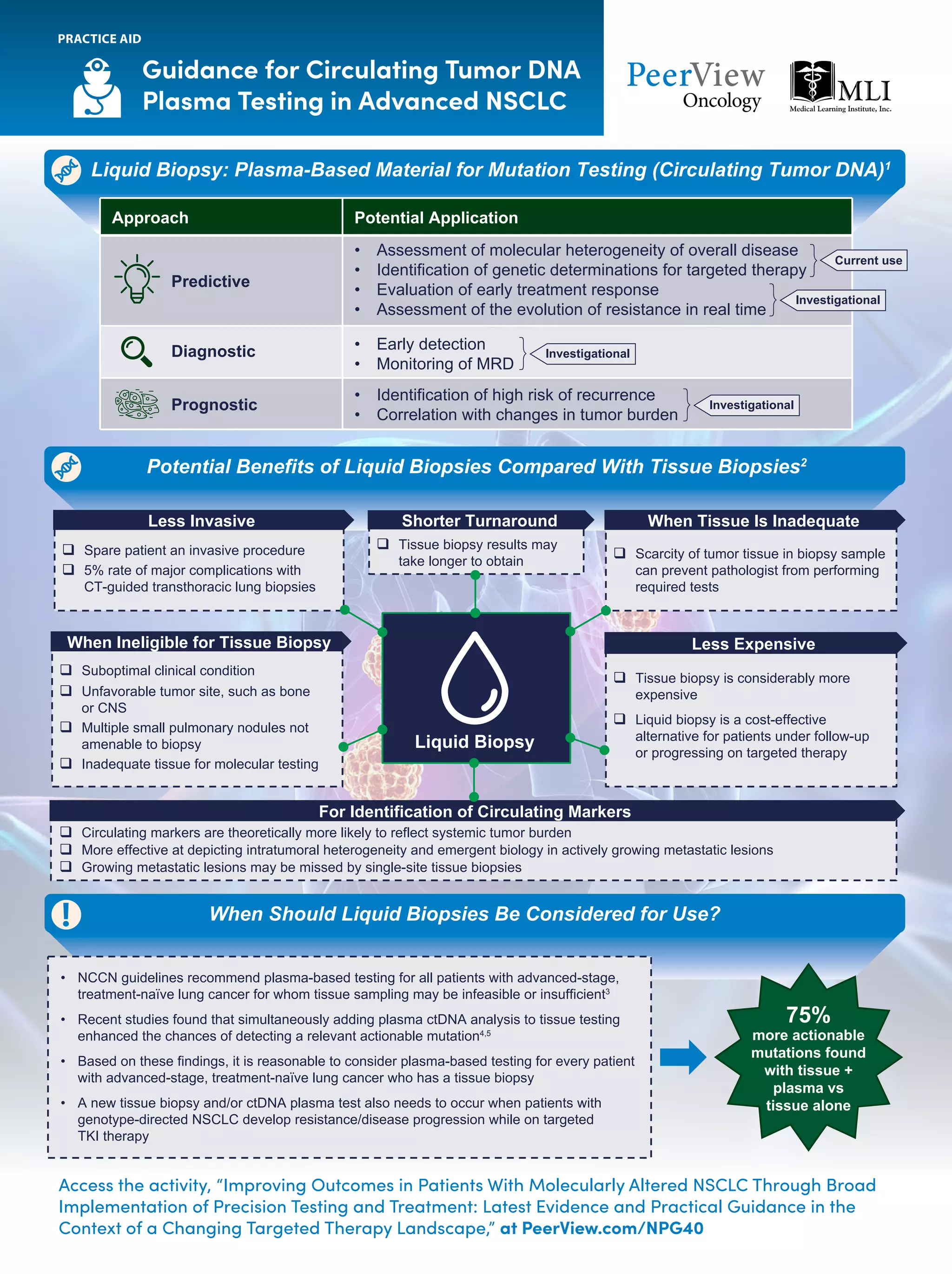
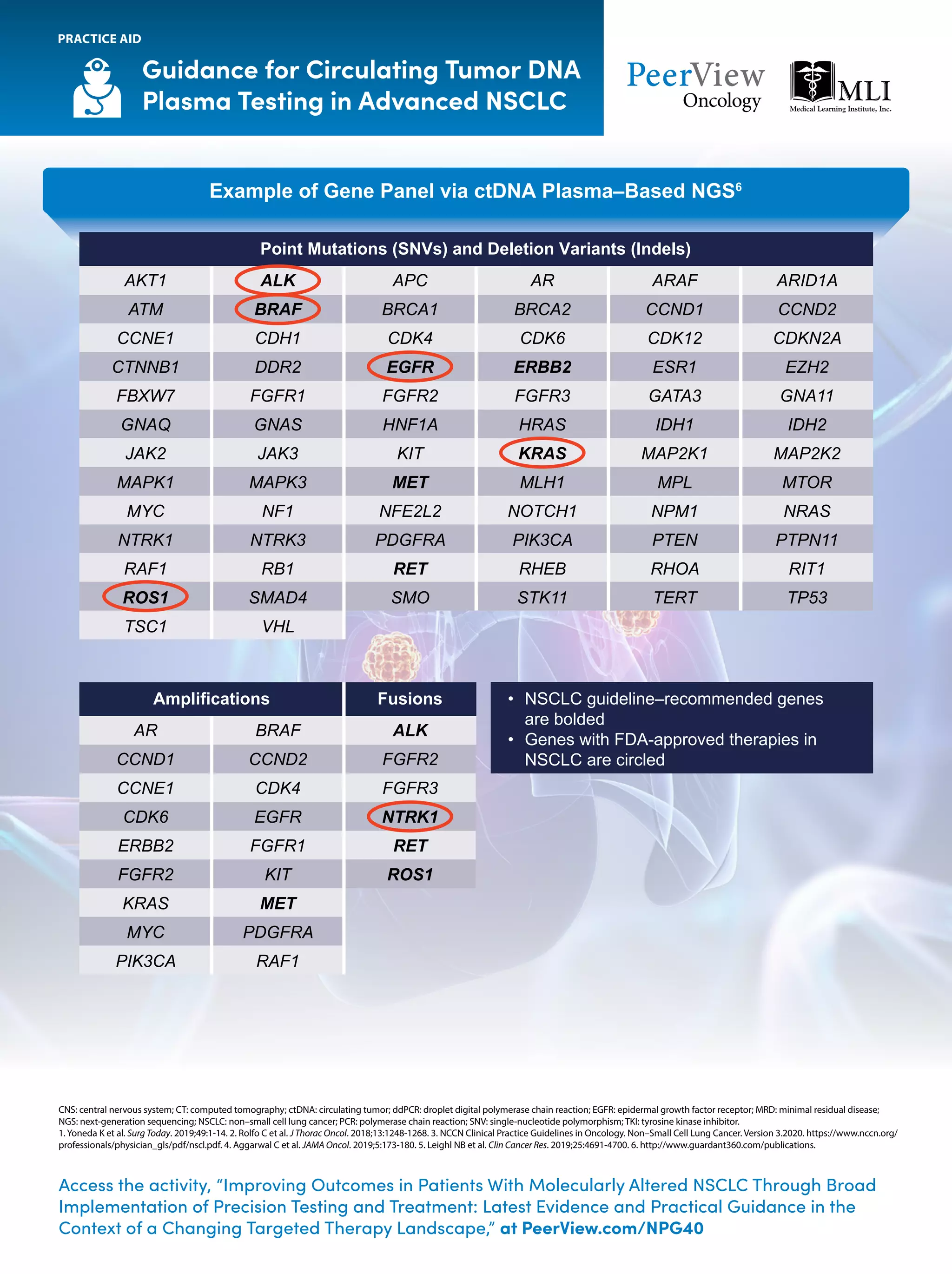
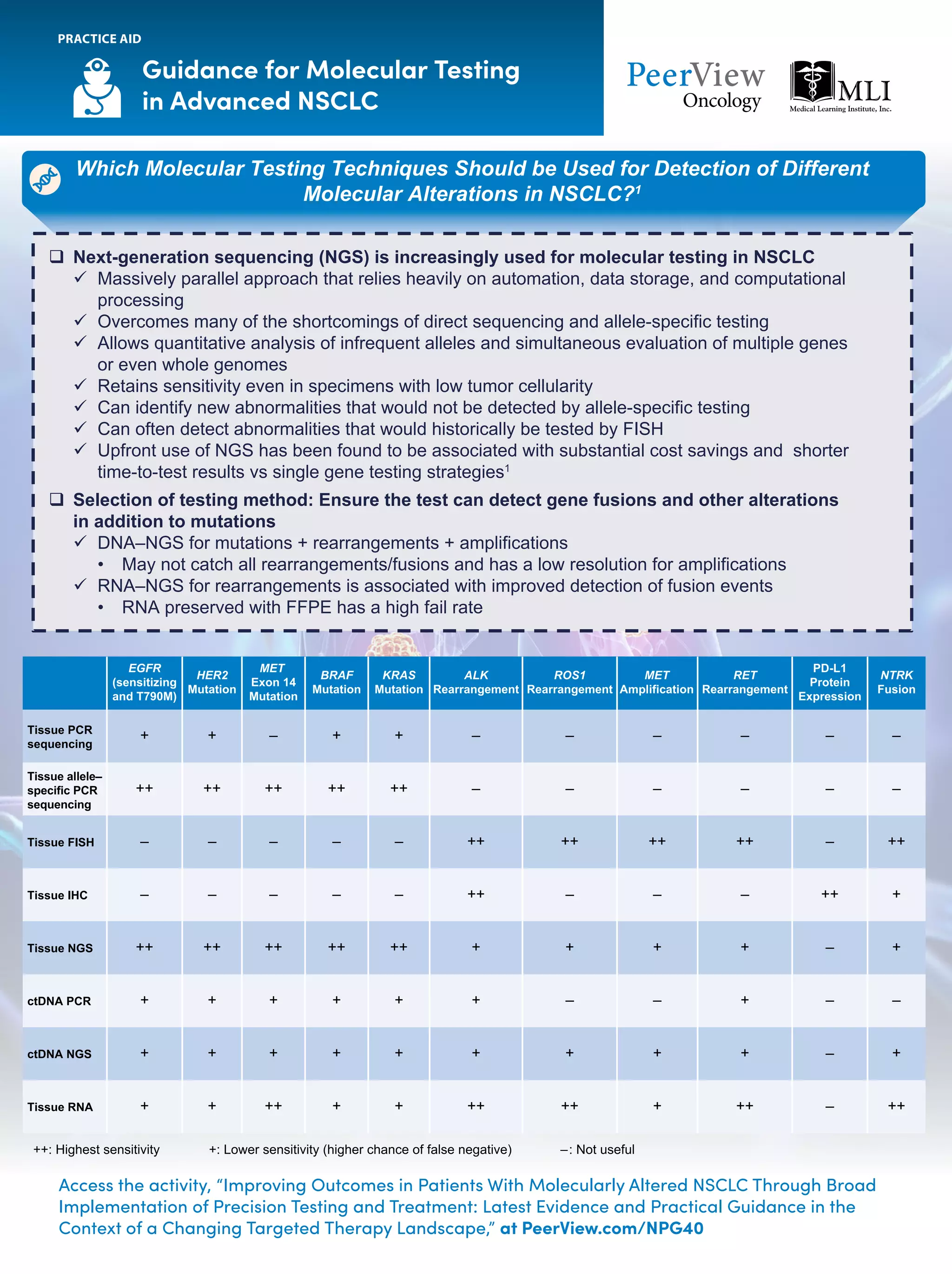
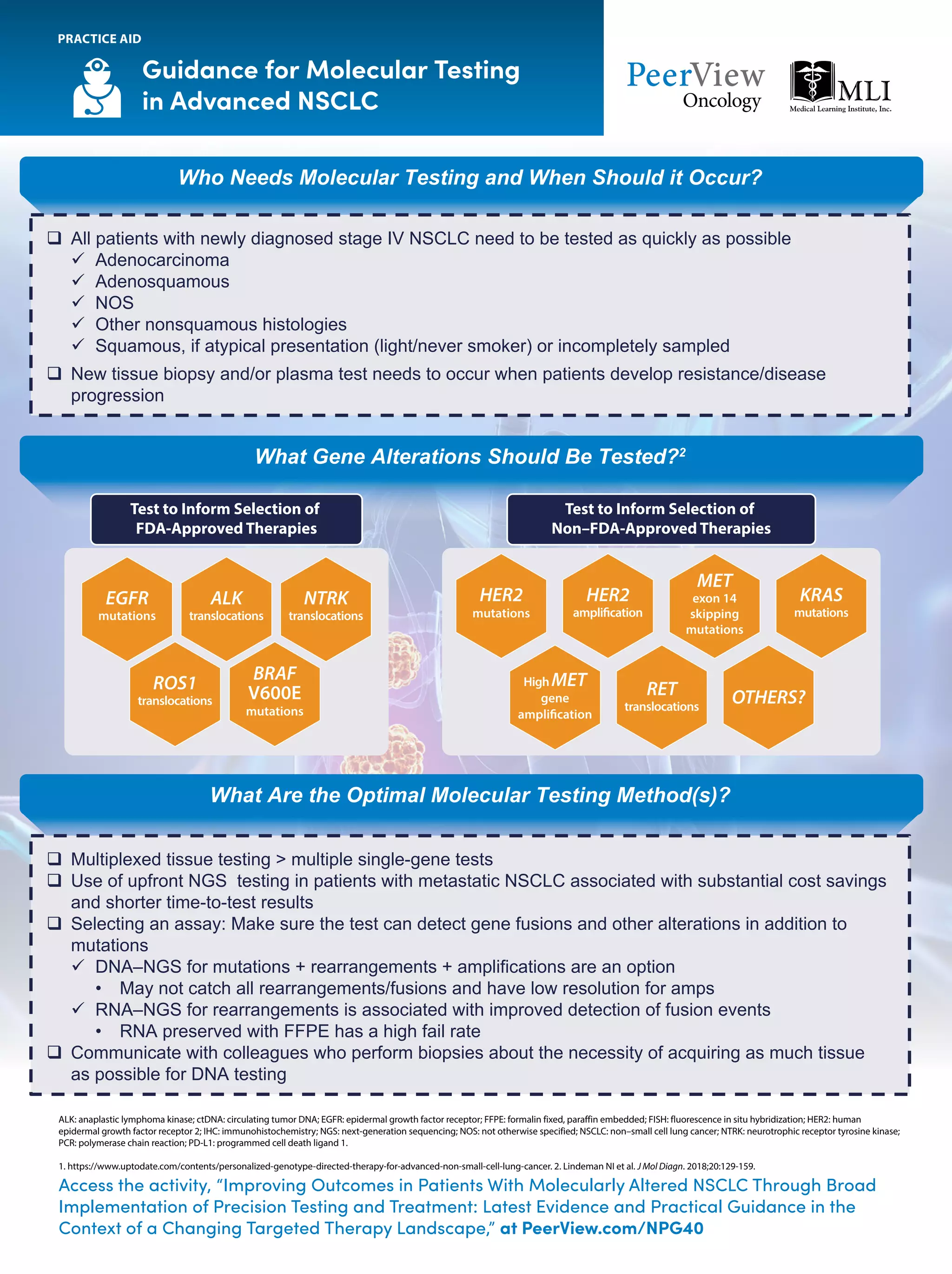
The document outlines key genomic alterations in non-small cell lung cancer (NSCLC) and their associated targeted therapies, highlighting FDA-approved options and the importance of molecular testing. It emphasizes the role of circulating tumor DNA (ctDNA) in liquid biopsies as a cost-effective alternative to tissue biopsies for monitoring treatment response and detecting mutations. Recommendations for molecular testing techniques and the necessity of testing all patients with stage IV NSCLC as quickly as possible are also provided.
![NSCLC: Key Genomic Alterations and Current
or Emerging Matched Targeted Therapies1
a
Approved by the FDA. b
FDA-designated Priority Review status for New Drug Application. c
FDA-designated Breakthrough Therapy status. d
FDA-designated Fast Track status.
ALK: anaplastic lymphoma kinase; DDR2: discoidin domain receptor tyrosine kinase 2; EGFR: epidermal growth factor receptor; FGFR: fibroblast growth factor receptor; HER2: human epidermal growth factor receptor 2; MAP2K1: mitogen-activated protein kinase kinase 1;
NSCLC: non–small cell lung cancer; NTRK1: neurotrophic receptor tyrosine kinase 1; PIK3CA: phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha.
1. Adapted from http://mlabs.umich.edu/molecular-diagnostics/moldx/lungcancer.
Access the activity, “Improving Outcomes in Patients With Molecularly Altered NSCLC Through Broad Implementation
of Precision Testing and Treatment: Latest Evidence and Practical Guidance in the Context of a Changing Targeted
Therapy Landscape,” at PeerView.com/NPG40
PRACTICE AID
NTRK1 Fusions
Larotrectiniba
Entrectiniba
Selitrectinib
Repotrectinib
ALK Rearrangements
Crizotinib,a
Ceritinib,a
Alectinib,a
Brigatinib,a
Lorlatiniba
ROS1 Rearrangements
Crizotiniba
Entrectiniba
Ceritinib
Lorlatinib
Repotrectinibd
BRAF V600E Mutations
Dabrafenib + Trametiniba
Vemurafenib
Dabrafenib
KRAS G12C Mutations
AMG 510d
MRTX849
RET Fusions
Selpercatinibb
Pralsetinibc
EGFR Exon 20 Mutations
TAK-788
Poziotinib
JNJ-6372c
EGFR Mutations
Osimertinib,a
Afatinib,a
Dacomitinib,a
Erlotinib,a
Gefitiniba
Erlotinib + Ramucirumabb
Gefitinib + Chemotherapy
Osimertinib + Chemotherapy
MET Exon 14 Skipping Mutations
Capmatinib
Tepotinibc
HER2 Mutations
T-DM1
[Fam-] Trastuzumab Deruxtecan
Poziotinib
Pyrotinib
MAP2K1
AKT1
PIK3CA
FGFR3
FGFR2
FGFR1
DDR2
NRAS
Clinically
Important Genomic
Alterations
in NSCLC](https://image.slidesharecdn.com/pvipracticeaidsnpg-200610193508/75/Improving-Outcomes-in-Patients-With-Molecularly-Altered-NSCLC-Through-Broad-Implementation-of-Precision-Testing-and-Treatment-Latest-Evidence-and-Practical-Guidance-in-the-Context-of-a-Changing-Targeted-Therapy-Landscape-1-2048.jpg)