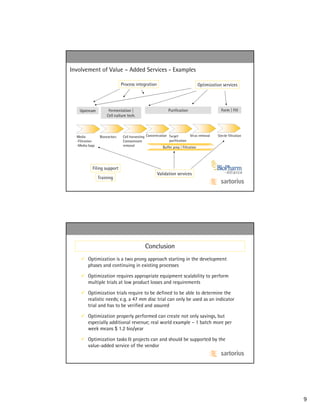
The document discusses optimizing biopharmaceutical processing through a two-pronged approach of optimization during development and scaling phases as well as of existing production processes. It provides examples of how optimization can reduce costs through lower product losses, validation efforts, and equipment sizing while also increasing revenue from higher throughput and capacity utilization. The document emphasizes that optimization requires equipment scalability and validation support services to determine realistic savings and revenue opportunities.



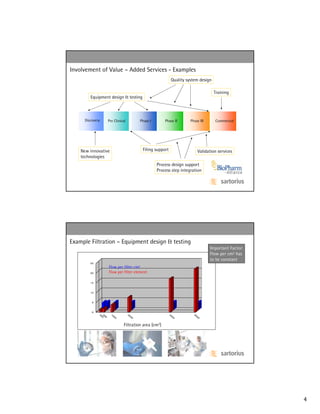


![Examples – Savings by Process Optimization of Tanks/Mixing
Disposable Bags
(low or no capital investment, low storage space needs,
no cleaning, i.e. no down-time, higher capacity utilization,
multi-product use posibilities, less man hours, less cleaning
solution costs - one tank cleaning can take up to 6 hours
and costs up to $ 10,000 per tank total)
Disposable Mixing
(lower capital investment and storage needs due to the
elimination of multiple sets of tanks (one for use, one in
cleaning), no cleaning , i.e. no down-time, higher capacity
utilization, multi-product use posibilities, less man hours, less
cleaning solution costs – one more batch per week due to
higher capacity utilization adds tens of millions of revenue)
Validation Support – Example – Extractable Testing
Any polymeric system requires
leachable/extractable testing
with the actual product or a Gamma-Bag Extraction with DI-Water at 50°C
model solvent under process 22
Acetic Acid
22
TOC [mg/l] / Conductivity [µS/cm ]
20 20
conditions
TOC
18 18
pH
pH / Acetic Acid [mg/ l]
16 16
Conductivity
14 14
12 12
10 10
8 8
6 6
4 4
2 2
Value-Added Service 0
Blank 24h 50°C 48h 50°C 120h 50°C
0
Extraction Time and Temperature
CONFIDENCE®
7](https://image.slidesharecdn.com/pdavirtualtrainingoptimizingprocesses032006-12861949430317-phpapp02/85/PDA-Virtual-Training-Optimizing-Processes-03-2006-7-320.jpg)