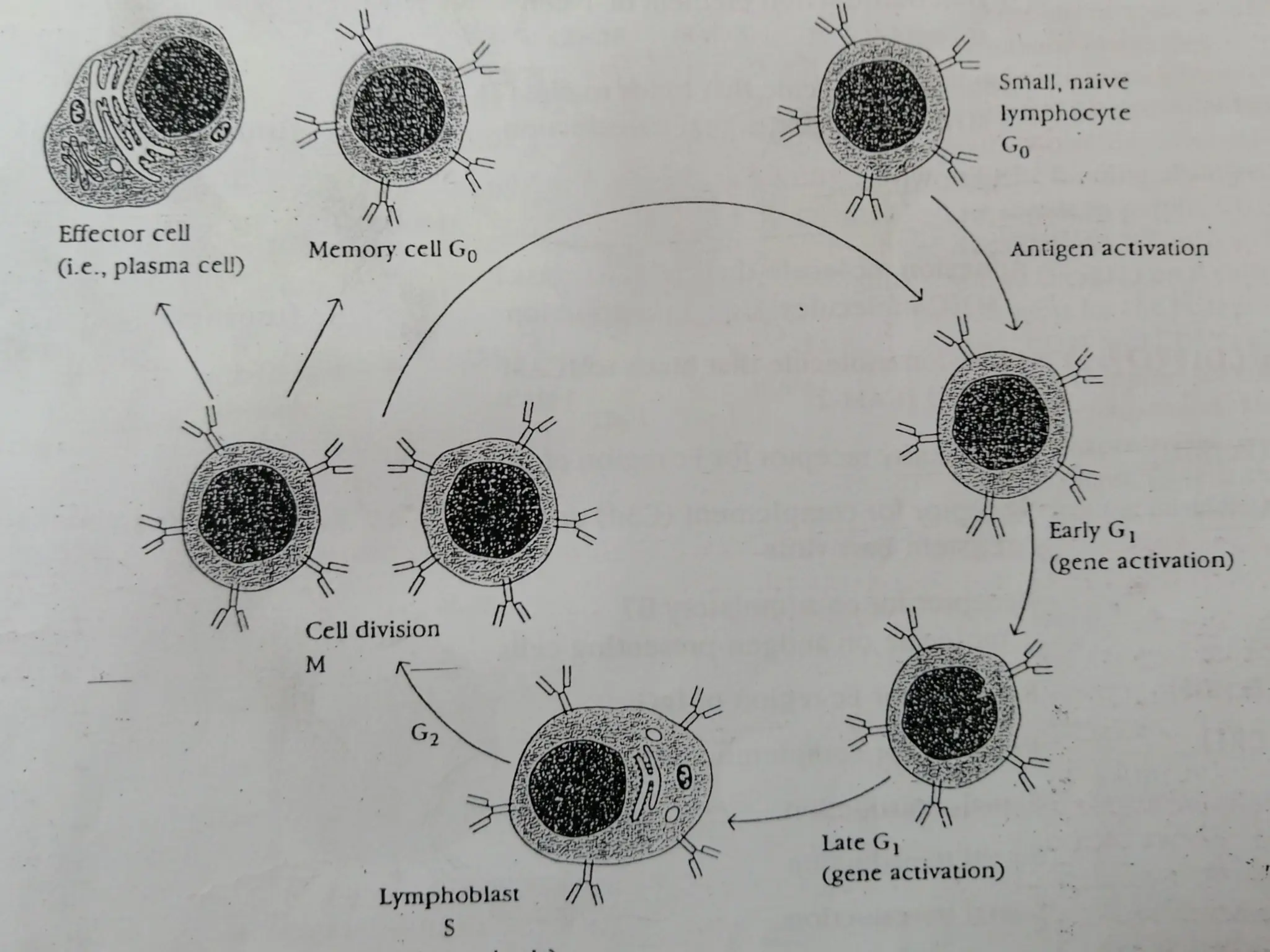
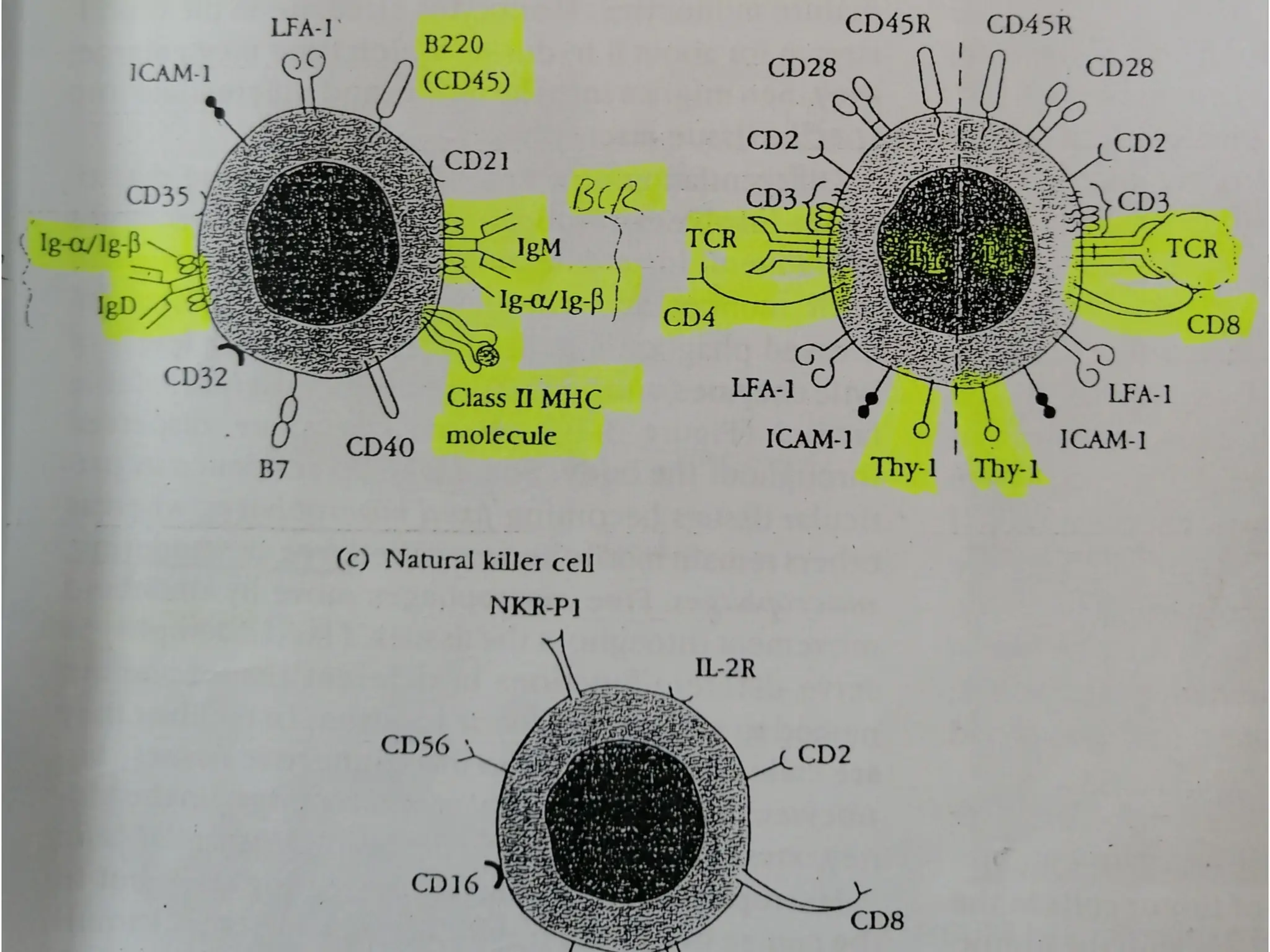
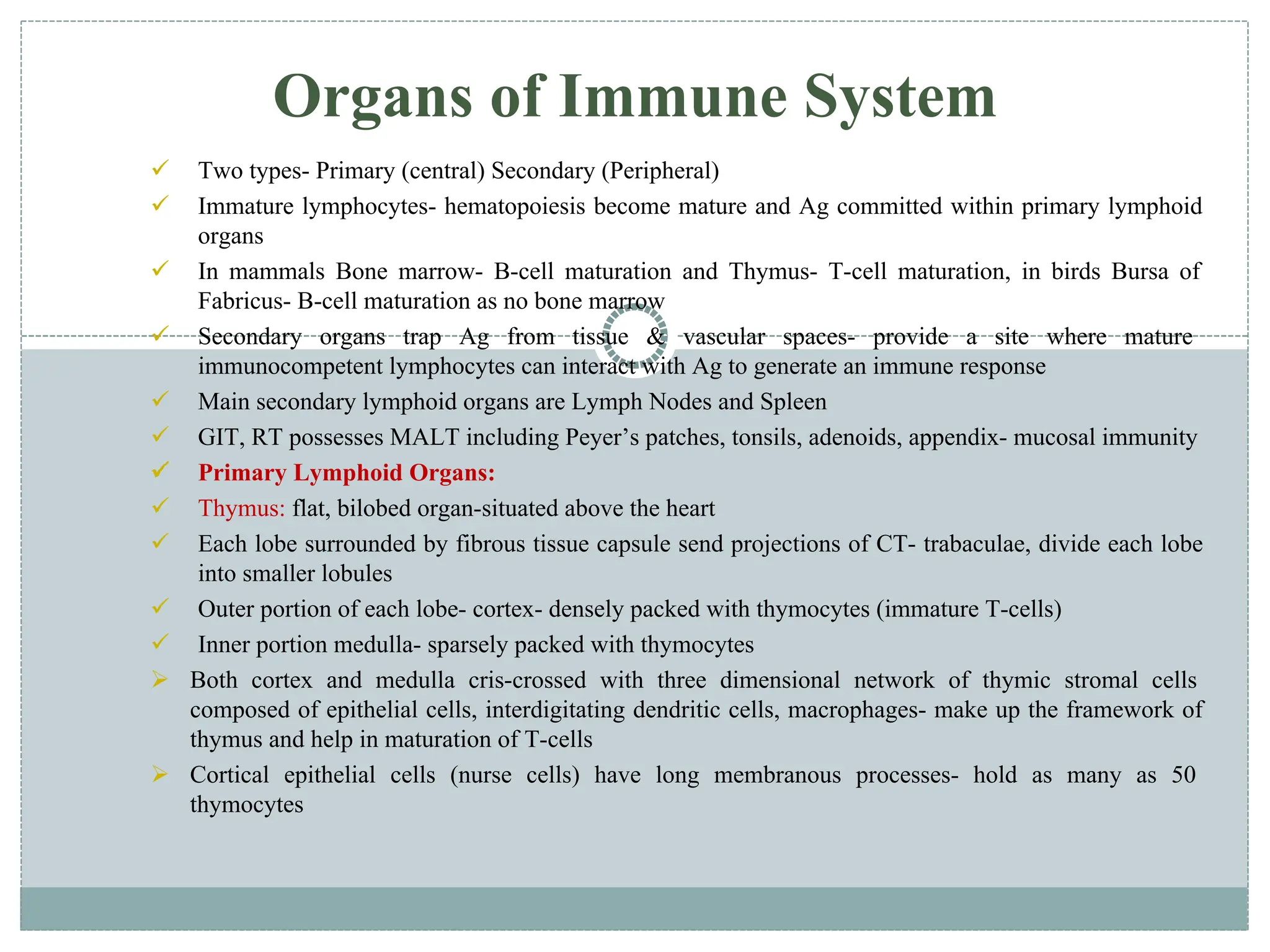
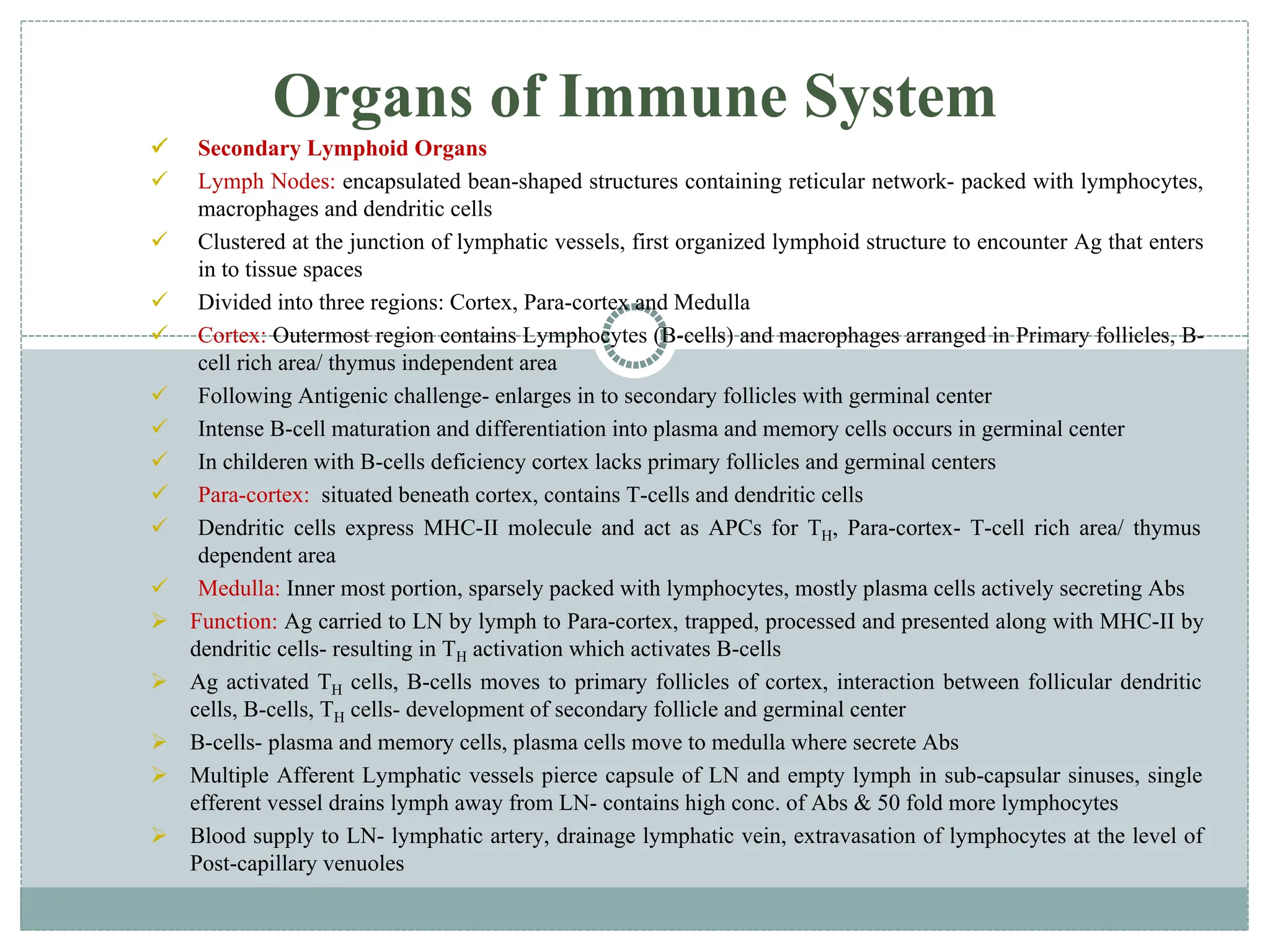
The document provides an extensive overview of the immune system, detailing the types of immunity (innate and acquired), the various cells and organs involved, and how the body defends against pathogens. It explains the roles of lymphocytes, macrophages, and other immune cells, as well as the processes of maturation and activation within primary and secondary lymphoid organs. Additionally, it describes the mechanisms of both humoral and cell-mediated immune responses, illustrating how the immune system is structured to recognize and eliminate pathogens effectively.