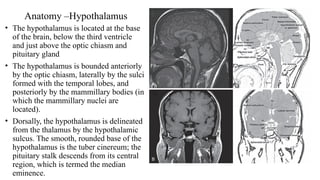
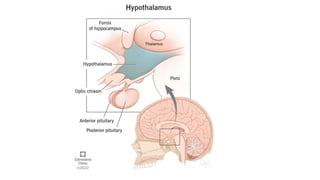
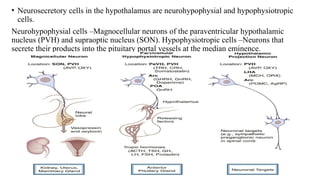
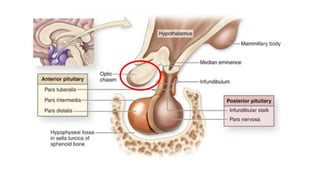
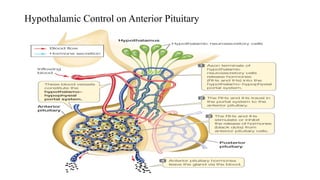
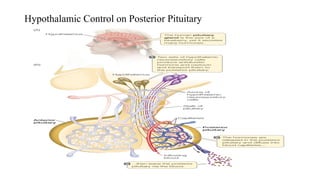
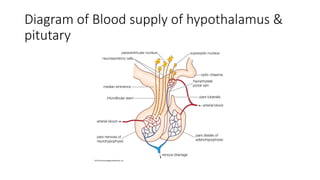
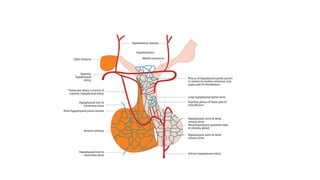
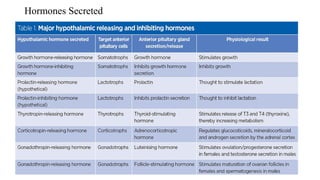
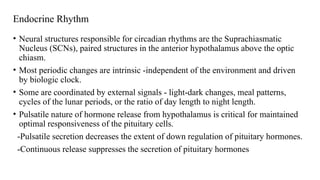
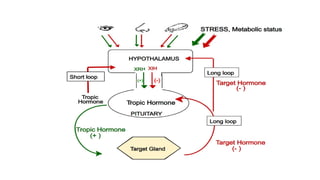
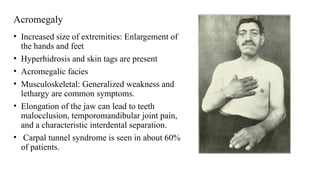
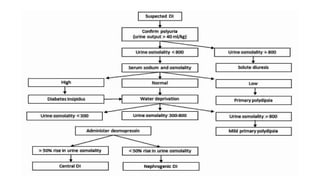
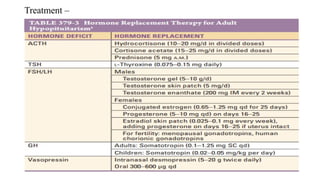
The document discusses the hypothalamic-pituitary axis, its anatomy, hormones, and clinical implications such as acromegaly, central diabetes insipidus, and central hypothyroidism. It emphasizes the role of the hypothalamus in regulating various hormonal functions essential for survival, and outlines treatments for disorders stemming from dysfunction in this axis. Key topics include feedback mechanisms, endocrine rhythms, and management strategies for conditions like hyperprolactinemia and functional hypothalamic amenorrhea.