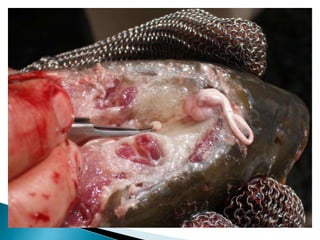
The document discusses the hypophysation technique for inducing breeding in fish. It involves removing the pituitary gland from donor fish, preparing an extract from the gland, and injecting the extract into recipient fish to induce spawning. The technique was first developed commercially in Brazil and was attempted in India in 1937 to induce breeding in Mrigal fish. The document provides details on selecting donor fish, removing and storing pituitary glands, preparing the extract, injecting recipients, and releasing induced donor fish.