Hypertension PPT.pptx
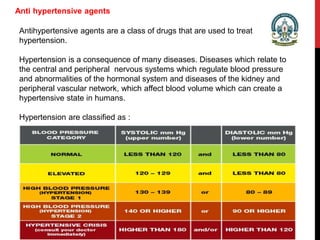
- 1. Anti hypertensive agents Antihypertensive agents are a class of drugs that are used to treat hypertension. Hypertension is a consequence of many diseases. Diseases which relate to the central and peripheral nervous systems which regulate blood pressure and abnormalities of the hormonal system and diseases of the kidney and peripheral vascular network, which affect blood volume which can create a hypertensive state in humans. Hypertension are classified as :
- 2. Classification of Antihypertensive drugs 1. Adrenergic receptors antagonist : Timolol 2. Angiotensin converting enzyme Inhibitors ( ACE – INHIBITORS) - Captopril , Lisinopril, Enalapril, Benazepril, Quinapril. 3. Centrally acting adrenergic drugs : Methyl dopa, Clonidine hydrochloride, Guanabenz acetate. 4. Vasodilators : Sodium nitroprusside, Hydralazine hydrochloride, Diazoxide, Minoxidil 5. Adrenergic neuron blocking agents : Guanethidine, reserpine] 6. Diuretics : Furosemide, Thiazides 7. Calcium channel Blockers : Amlodipine, Felodipine, Verapamil
- 3. Adrenergic receptors antagonist ( β – Blockers) Beta –blockers are the most widely employed antihypertenives and the treatment of Glaucoma. Aryl oxy propyl amines. Timolol : CC(C)(C)NCC(COC1=NSN=C1N2CCOCC2)O GI absorption High BBB permeant No P-gp substrate Yes CYP1A2 inhibitor No CYP2C19 inhibitor No CYP2C9 inhibitor No CYP2D6 inhibitor No CYP3A4 inhibitor No Log Kp (skin permeation) -8.24 cm/s
- 4. Mechanism of action : It competes with adrenergic neurotransmitters such as catecholamines for binding at Beta – 1 adrenergic receptors in the heart and vascular smooth muscle and Beta -2 receptors in the bronchial and vascular smooth muscles. Beta-2 Adrenergic Receptors (B2ARs) are a type of G Protein-Coupled Receptor (GPCR). GPCRs are the largest family of integral membrane proteins in the human body with over 1000 unique Isoforms. B2AR is activated by hormone ligands like adrenaline (epinephrine) and noradrenaline and plays a critical role in cardiovascular and pulmonary physiology. Binding of adrenaline by B2AR causes a sympathetic nervous system response like the well-known “fight or flight response”, resulting in an increased heart rate, pupil dilation, rapid energy mobilization and diversion of blood to skeletal muscle. More precisely, upon binding a ligand, B2AR activates Adenylyl cyclase through interaction with B2ARs C-terminus. Adenylyl cyclase subsequently converts ATP into cAMP, which functions as a downstream signaling molecule activating effectors like cAMP-dependent protein kinases, resulting in various bodily responses.[ Uses : It is in oral form it is used to treat hypertension
- 5. Angiotensin Converting Enzyme inhibitors ( ACE inhibitors)
- 6. RENIN ANGIOTENSIN ALDOSTERONE SYSTEM Several pathophysiologic factors are involved in the relationship between HTN and the other components of the CMS, including inappropriate activation of the renin angiotensin aldosterone system (RAAS), oxidative stress, and inflammation. The renin – angiotensin system is a hormonal system that plays a central role in the control of sodium excretion and body fluid volume. It interacts closely with the sympathetic nervous system and aldosterone secretion in the regulation of the Blood pressure. 1. Renin is an aspartyl Protease cleaves the Leu –Val bond from the aspartic acid end of the angiotensinogen polypetide molecule to release the decapeptide angiotensin –I. 2. The cleavage of dipeptide from the carboxy terminal of angiotensin –I by angiotensin Converting enzyme to form octa peptide angiotensin –II. 3. Angiotensin –II is a potent vasoconstrictor that increases peripheral resistance through a variety of mechanisms. 4. Angiotensin –II causes a slow pressor response resulting in long –term stabilization of arterial Blood pressure. 5. This long term effect is accomplished by the regulation of renal function. 6. Angiotensin –II increases sodium reabsorption in the proximal tubule. 7. It also alters renal hemodynamics and causes the release of aldosterone from the adrenal cortex. 8. Angitensin –III is formed by removal of the N –terminal aspartate residue of the angiotensin – II, a reaction catalyzed by glutamyl amino peptidase.
- 7. Renin Angiotensin system of blood Pressure control
- 8. Renin, also known as angiotensinogenase, is an aspartyl protease and belongs to the protein family peptidase A1. Aspartyl proteases are endopeptidases that typically use two aspartate residues in the active site to specifically cleave peptide substrates using an acid-base hydrolysis mechanism. Mature renin circulates in the blood stream and contains 340 amino acid residues and has a mass of approximately 37 kDa. The function of renin is to cleave angiotensinogen to produce angiotensin I. Factor XII – coagulation factor
- 9. The regulation action of the RAAS system is controlling sodium and potassium balance and arterial blood pressure is modified by vasodilators called kinins. Callidin which is converted to bradykinin is activated in plasma by noxious influences to act on a kinin, callidin which is converted to bradykinin by tissue enzymes.
- 10. ACE inhibitors : These compounds can be sub –classified in to three groups based on their chemical composition. 1.Sulfhydryl – containing inhibitors : Captopril 2. Dicarboxylate containing Inhibitors : Enalapril, lisinopri;,Benazepril. 3.Phosphonate containing inhibitors : Fosinopril SAR of ACE inhibitors : 1. The N-ring must contain a carboxylic acid to mimic the C-terminal carboxylate of ACE substrates. 2. Large hydrophobic heterocyclic rings ( The N- ring) increase the potency and alter the Pharmacokinetic properties. 3. The zinc binding groups can be either sulfhydryl (A), carboxylic acid (B) or a Phosphinic acid ( C). 4. Sulfhydryl containing compounds produce high incidence of skin rash and taste disturbances. 5. Sulfhydryl – containing compounds to form dimers and disulfides which may shorten duration of action.
- 11. 7. Compounds that bind to zinc through either a carboxylate or Phosphine mimic the peptide hydrolysis transition state and enhance binding. 8. Esterification of the carboxylate or phosphine produces an norally bioavailable pro drug. 9. X is usually methyl to mimic the side chain of alanine. With in the dicarboxylate series , when X equals n- butyl amine this produces a compound that does not require prodrug for oral activity. 10.Optimum activity occurs when stereochemistry of inhibitor is consistent with L- amino acid sterochemistry present in normal substances. MOA : The ACE inhibitors attenuate the effects of the renin angiotensin system by inhibiting the conversion of angiotensin –I to Angiotensin –II. They also inhibit the conversion of stimulate prostaglandin biosynthesis..
- 13. Sulfhydryl – containing inhibitors : (2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolidine-2-carboxylic acid Captopril is a potent, competitive inhibitor of angiotensin-converting enzyme (ACE), the enzyme responsible for the conversion of angiotensin I (ATI) to angiotensin II (ATII). ATII regulates blood pressure and is a key component of the renin-angiotensin-aldosterone system (RAAS). Captopril may be used in the treatment of hypertension.
- 14. Pharmacodynamics Captopril, an ACE inhibitor, antagonizes the effect of the RAAS. The RAAS is a homeostatic mechanism for regulating hemodynamics, water and electrolyte balance. During sympathetic stimulation or when renal blood pressure or blood flow is reduced, renin is released from the granular cells of the juxtaglomerular apparatus in the kidneys. In the blood stream, renin cleaves circulating angiotensinogen to ATI, which is subsequently cleaved to ATII by ACE. ATII increases blood pressure using a number of mechanisms. First, it stimulates the secretion of aldosterone from the adrenal cortex. Aldosterone travels to the distal convoluted tubule (DCT) and collecting tubule of nephrons where it increases sodium and water reabsorption by increasing the number of sodium channels and sodium- potassium ATPases on cell membranes. Second, ATII stimulates the secretion of vasopressin (also known as antidiuretic hormone or ADH) from the posterior pituitary gland. ADH stimulates further water reabsorption from the kidneys via insertion of aquaporin-2 channels on the apical surface of cells of the DCT and collecting tubules. Third, ATII increases blood pressure through direct arterial vasoconstriction. Stimulation of the Type 1 ATII receptor on vascular smooth muscle cells leads to a cascade of events resulting in myocyte contraction and vasoconstriction. In addition to these major effects, ATII induces the thirst response via stimulation of hypothalamic neurons. ACE inhibitors inhibit the rapid conversion of ATI to ATII and antagonize RAAS-induced increases in blood pressure. ACE (also known as kininase II) is also involved in the enzymatic deactivation of bradykinin, a vasodilator. Inhibiting the deactivation of bradykinin increases bradykinin levels and may sustain its effects by causing increased vasodilation and decreased blood pressure.
- 16. GI absorption High BBB permeant No P-gp substrate No CYP1A2 inhibitor No CYP2C19 inhibitor No CYP2C9 inhibitor No CYP2D6 inhibitor No CYP3A4 inhibitor No Log Kp (skin permeation) -7.38 cm/s
- 17. Uses : For the treatment of essential or reno vascular hypertension. Adverse effects : Skin rashes, and taste disturbances.. SAR of ACE inhibitors : 1. The N- ring must contain a carboxylic acid to mimic the C-terminal carboxylate of ACE susbstrates. 2. The zinc binding groups can be either sulhydryl (A). A carboxylic acid (B) or a phosphinic acid (C). 3. The sulfhydryl group shows superior binding to zinc (the side chain mimicking the Phe in carboxylate and phosphinic acid compounds partially compensates for the lack of a sulfhydryl group). 4. Sulfhydryl – containing compounds produce high incidence of skin rash and taste disturbances. 5. Sulfhydryl – containing compounds can form dimers and disulfides which may shorten duration of action. 6. Compounds that bind to zinc through either a carboxylate or phosphinate mimic the peptide hydrolysis transition state and enhances binding. 7. Esterification of the carboxylate or phosphinate produces an orally bioavailable prodrug. 8. X is usually methyl to mimic the side chain of alanine. 9. Optimum activity occurs when stereochemistry of inhibitor is consistent with L- amino acid stereochemistry present in normal substrates.
- 18. Enalapril O C H3 O NH N O O H O OC(=O)C1CCCN1C(=O)CNC(CC(=O)OCC)CCc1ccccc1 1-{2-[(1-ethoxy-1-oxo-5-phenylpentan-3- yl)amino]acetyl}pyrrolidine-2-carboxylic acid GI absorption High BBB permeant No P-gp substrate Yes CYP1A2 inhibitor No CYP2C19 inhibitor No CYP2C9 inhibitor No CYP2D6 inhibitor No CYP3A4 inhibitor No Log Kp (skin permeation) -8.79 cm/s
- 19. MOA : It is a long acting ACE inhibitor. It requires activation by hydrolysis of its ethyl ester to form the diacid enalaprilat. Uses : It is used as an renovascular hypertension and symptomatic congestive heart failure. Adv effects : Hypotension,headache, dizziness and fatigue. Lisinopril OC(=O)C1CCCN1C(=O)C(CCCCN)NC(CCc1ccccc1)C(=O)O
- 20. Formula C21H31N3O5 Molecular weight 405.49 g/mol Num. heavy atoms 29 Num. arom. heavy atoms 6 Fraction Csp3 0.57 Num. rotatable bonds 13 Num. H-bond acceptors 7 Num. H-bond donors 4 Molar Refractivity 112.66 TPSA 132.96 Ų GI absorption High BBB permeant No P-gp substrate Yes CYP1A2 inhibitor No CYP2C19 inhibitor No CYP2C9 inhibitor No CYP2D6 inhibitor No CYP3A4 inhibitor No Log Kp (skin permeation) -10.80 cm/s
- 22. Uses : For the treatment of hypertension and symptomatic congestive heart failure. Benazepril Hydrochloride [H][C@@]1(CCC2=CC=CC=C2N(CC(O)=O)C1=O)N[C@@H](CCC1=CC=CC=C1)C(=O) OCC 2-[(3S)-3-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}- 2-oxo-2,3,4,5-tetrahydro-1H-1-benzazepin-1-yl]acetic acid InChI=1S/C24H28N2O5/c1-2-31-24(30)20(14-12-17-8-4-3-5-9-17)25-19-15-13-18-10-6- 7-11-21(18)26(23(19)29)16-22(27)28/h3-11,19-20,25H,2,12-16H2,1H3,(H,27,28)/t19- ,20-/m0/s1
- 23. Renin Formula C24H28N2O5 Molecular weight 424.49 g/mol Num. heavy atoms 31 Num. arom. heavy atoms 12 Fraction Csp3 0.38 Num. rotatable bonds 10 Num. H-bond acceptors 6 Num. H-bond donors 2 Molar Refractivity 120.24 TPSA 95.94 Ų
- 24. GI absorption High BBB permeant No P-gp substrate Yes CYP1A2 inhibitor No CYP2C19 inhibitor No CYP2C9 inhibitor No CYP2D6 inhibitor Yes CYP3A4 inhibitor Yes Log Kp (skin permeation) -7.99 cm/s Benazepril ADME Uses : For the treatment of hypertension. Adv.effects : Headache, dizziness, fatigue, postural dizziness, nausea and cough.
- 26. Formula C25H30N2O5 Molecular weight 438.52 g/mol Num. heavy atoms 32 Num. arom. heavy atoms 12 Fraction Csp3 0.40 Num. rotatable bonds 11 Num. H-bond acceptors 6 Num. H-bond donors 2 Molar Refractivity 124.49 TPSA 95.94 Ų MOA : Quinaprilat the principle active metabolite of quinapril competes with AT1 for binding to ACE and inhibits and enzymatic proteolysis of AT1 to AT2. Decreasing AT2 levels in the body decreases blood pressire by inhibiting the presence effects of AT2.
- 27. PAINS 0 alert Brenk 0 alert Leadlikeness No; 2 violations: MW>350, Rotors>7 Synthetic accessibility GI absorption High BBB permeant No P-gp substrate Yes CYP1A2 inhibitor No CYP2C19 inhibitor No CYP2C9 inhibitor No CYP2D6 inhibitor No CYP3A4 inhibitor Yes Log Kp (skin permeation) -8.09 cm/s Pan-assay interference compounds (PAINS) Uses : For the treatment of hypertension and as an adjunct therapy in the treatment of congestive heart failure. Adv.effects : Dizziness, cough, chest pain, dyspnea, nausea and vomiting
- 28. Dicarboxylate – containing inhibitors The phosphinic acid is capable of binding to ACE in a manner similar to enalapril. The interaction of the zinc atom with the phosphinic acid with sulfhydryl and carboxylate groups Fosinopril: Fosinopril is a phosphinic acid-containing ester prodrug that belongs to the angiotensin-converting enzyme (ACE) inhibitor class of medications. It is rapidly hydrolyzed to fosinoprilat, its principle active metabolite. Fosinoprilat inhibits ACE, the enzyme responsible for the conversion of angiotensin I (ATI) to angiotensin II (ATII). ATII regulates blood pressure and is a key component of the renin-angiotensin-aldosterone system (RAAS). Fosinopril may be used to treat mild to moderate hypertension, as an adjunct in the treatment of congestive heart failure, and to slow the rate of progression of renal disease in hypertensive individuals with diabetes mellitus and microalbuminuria or overt nephropathy.
- 30. Fosinopril is an ester prodrug that hydrolyzes in the liver to fosinoprilat, the active metabolite form. Fosinoprilat then uses competitive inhibition to bind to ACE resulting in decreased formation of angiotensin II, reduced aldosterone concentrations, and diminished systemic vasoconstriction. Formula C30H46NO7P Molecular weight 563.66 g/mol Num. heavy atoms 39 Num. arom. heavy atoms 6 Fraction Csp3 0.70 Num. rotatable bonds 16 Num. H-bond acceptors 7 Num. H-bond donors 1 Molar Refractivity 157.56 TPSA 120.02 Ų
- 31. GI absorption Low BBB permeant No P-gp substrate Yes CYP1A2 inhibitor No CYP2C19 inhibitor No CYP2C9 inhibitor Yes CYP2D6 inhibitor No CYP3A4 inhibitor Yes Log Kp (skin permeation) -5.31 cm/s Uses : For treating mild to moderate hypertension.
- 33. Centrally Acting adrenergic drugs The use of agents that directly affect the peripheral component of the sympathetic nervous system represents to the treatment of hypertension. The second approach to modifying sympathetic influence on the cardiovascular system is through inhibition of reduction of CNS control of blood pressure. Methyl dopate hydrochloride. (2S)-2-amino-3-(3,4-dihydroxyphenyl)-2- methylpropanoic acid C[C@](N)(CC1=CC=C(O)C(O)=C1)C(O)=O
- 34. MOA : Methyl dopa on coonversion to alpha – methyl nor adrenaline which acts on α2- adrenergic receptors to inhibit the release of nor adrenaline, resulting in decreased sympathetic outflow from the CNS and activation of parasympathetic out flow. ADME : Formula C10H13NO4 Molecular weight 211.21 g/mol Num. heavy atoms 15 Num. arom. heavy atoms 6 Fraction Csp3 0.30 Num. rotatable bonds 3 Num. H-bond acceptors 5 Num. H-bond donors 4 Molar Refractivity 54.39 TPSA 103.78 Ų
- 35. GI absorption High BBB permeant No P-gp substrate No CYP1A2 inhibitor No CYP2C19 inhibitor No CYP2C9 inhibitor No CYP2D6 inhibitor No CYP3A4 inhibitor No Log Kp (skin permeation) -9.19 cm/s Uses : Management of moderate to severe hypertension. Adv.effects : 48 -72 hrs therapy and can dissapear with continued administration of the drug.
- 36. Synthesis :
- 37. Adr effects : Drowsiness, sedation, orthostatic hypotension, Mental depression, Night mares. Guanabenz acetate 2,6 dichlorobenzylidene) amino) guanidine monoacetate.
- 38. Guanabenz's antihypertensive effect is thought to be due to central alpha- adrenergic stimulation, which results in a decreased sympathetic outflow to the heart, kidneys, and peripheral vasculature in addition to a decreased systolic and diastolic blood pressure and a slight slowing of pulse rate. Chronic administration of guanabenz also causes a decrease in peripheral vascular resistance.
- 39. ADME : Water solubility -1.818 Numeric (log mol/L) Absorption Caco2 permeability 0.722 Numeric (log Papp in 10 -6 cm/s) Absorption Intestinal absorption (human) 89.803 Numeric (% Absorbed) Absorption Skin Permeability -2.815 Numeric (log Kp) Absorption P-glycoprotein substrate No Categorical (Yes/No) Absorption P-glycoprotein I inhibitor No Categorical (Yes/No) Absorption P-glycoprotein II inhibitor No Categorical (Yes/No)
- 40. Distribution VDss (human) 1.084 Numeric (log L/kg) Distribution Fraction unbound (human) 0.543 Numeric (Fu) Distribution BBB permeability -0.561 Numeric (log BB) Distribution CNS permeability -2.893 Numeric (log PS) Metabolism CYP2D6 substrate No Categorical (Yes/No) Metabolism CYP3A4 substrate No Categorical (Yes/No) Metabolism CYP1A2 inhibitior Yes Categorical (Yes/No) Metabolism CYP2C19 inhibitior No Categorical (Yes/No) Metabolism CYP2C9 inhibitior No Categorical (Yes/No) Metabolism CYP2D6 inhibitior Yes Categorical (Yes/No) Metabolism CYP3A4 inhibitior No Categorical (Yes/No)
- 41. Excretion Total Clearance 0.247 Numeric (log ml/min/kg) Excretion Renal OCT2 substrate Yes Categorical (Yes/No) Toxicity AMES toxicity Yes Categorical (Yes/No) Toxicity Max. tolerated dose (human) 0.316 Numeric (log mg/kg/day) Toxicity hERG I inhibitor No Categorical (Yes/No) Toxicity hERG II inhibitor No Categorical (Yes/No) Toxicity Oral Rat Acute Toxicity (LD50) 3.038 Numeric (mol/kg) Toxicity Oral Rat Chronic Toxicity (LOAEL) 1.283 Numeric (log mg/kg_bw/day) Toxicity Hepatotoxicity No Categorical (Yes/No) Toxicity Skin Sensitisation Yes Categorical (Yes/No) Toxicity T.Pyriformis toxicity 0.684 Numeric (log ug/L) Toxicity Minnow toxicity 1.442 Numeric (log mM)
- 42. Pharmacodynamics Guanabenz, a centrally acting α-2 adrenergic agonist, is indicated for treatment of hypertension. Adverse effects : Clonidine Vasodilators : Vasodilators drugs relax the smooth muscle in blood vessels which causes the vessels to dilate. Dilation of arterial vessels leads to a reduction in systemic vascular resistance which leads to a fall in arterial blood pressure. Draw backs of use of vasodilators : 1. Vasodilators can lead to a baro-receptor mediated reflex stimulation of the heart from systemic vasodilation and arterial pressure reduction. 2.They can impair the normal baro –receptor mediated reflex vasoconstriction when a person stands up which can lead to orthostatic hypotension and syncope on standing. 3. They can lead to renal retention of sodium and water increasing blood volume and cardiac output.
- 43. Antihypertensive agents that produce vasodilation of smooth muscle can be divided in to two categories : 1. Direct acting : Hydralazine hydrochloride, sodium nitroprusside, potassium channel openers, calcium channel – blocking agents. 2. Indirect acting : Reserpine, alpha – adrenergic antagonists – Prazosin hydrochloride, ACE inhibitors and angiotensin –II receptor antagonists such as saralysin. Sodium Nitroprusside O=N[Fe--](C#N)(C#N)(C#N)(C#N)C#N Nitroprusside a powerful vasodilator relaxes the vascular smooth muscle and produce consequent dilatation of peripheral arteries and veins. Other smooth muscle (e.g., uterus, duodenum) is not affected. Sodium nitroprusside is more active on veins than on arteries.
- 44. One molecule of sodium nitroprusside is metabolized by combination with hemoglobin to produce one molecule of cyanmethemoglobin and four CN- ions; methemoglobin, obtained from hemoglobin, can sequester cyanide as cyanmethemoglobin; thiosulfate reacts with cyanide to produce thiocyanate; thiocyanate is eliminated in the urine; cyanide not otherwise removed binds to cytochromes. Cyanide ion is normally found in serum; it is derived from dietary substrates and from tobacco smoke. Cyanide binds avidly (but reversibly) to ferric ion (Fe+++), most body stores of which are found in erythrocyte methemoglobin (metHgb) and in mitochondrial cytochromes. When CN is infused or generated within the bloodstream, essentially all of it is bound to methemoglobin until intraerythrocytic methemoglobin has been saturated. Sodium nitroprusside is further broken down in the circulation to release nitric oxide (NO), which activates guanylate cyclase in the vascular smooth muscle. This leads to increased production of intracellular cGMP, which stimulates calcium ion movement from the cytoplasm to the endoplasmic reticulum, reducing the level of available calcium ions that can bind to calmodulin. This ultimately results in vascular smooth muscle relaxation and vessel dilation.
- 45. Water solubility -2.596 Numeric (log mol/L) Absorption Caco2 permeability 0.056 Numeric (log Papp in 10 - 6 cm/s) Absorption Intestinal absorption (human) 78.855 Numeric (% Absorbed) Absorption Skin Permeability -3.18 Numeric (log Kp) Absorption P-glycoprotein substrate Yes Categorical (Yes/No) Absorption P-glycoprotein I inhibitor No Categorical (Yes/No) Absorption P-glycoprotein II inhibitor No Categorical (Yes/No)
- 46. VDss (human) -0.604 Numeric (log L/kg) Distribution Fraction unbound (human) 0.669 Numeric (Fu) Distribution BBB permeability -0.755 Numeric (log BB) Distribution CNS permeability -3.076 Numeric (log PS) Distribution VDss (human) -0.604 Numeric (log L/kg) Distribution Fraction unbound (human) 0.669 Numeric (Fu) Distribution BBB permeability -0.755 Numeric (log BB) Distribution CNS permeability -3.076 Numeric (log PS)
- 47. Metabolism CYP2D6 substrate No Categorical (Yes/No) Metabolism CYP3A4 substrate No Categorical (Yes/No) Metabolism CYP1A2 inhibitior No Categorical (Yes/No) Metabolism CYP2C19 inhibitior No Categorical (Yes/No) Metabolism CYP2C9 inhibitior No Categorical (Yes/No) Metabolism CYP2D6 inhibitior No Categorical (Yes/No) Metabolism CYP3A4 inhibitior No Categorical (Yes/No)
- 48. Excretion Total Clearance 1.902 Numeric (log ml/min/kg) Excretion Renal OCT2 substrate No Categorical (Yes/No) Toxicity AMES toxicity No Categorical (Yes/No) Toxicity Max. tolerated dose (human) 0.753 Numeric (log mg/kg/day) Toxicity hERG I inhibitor No Categorical (Yes/No) Toxicity hERG II inhibitor No Categorical (Yes/No) Toxicity Oral Rat Acute Toxicity (LD50) 3.707 Numeric (mol/kg) Toxicity Oral Rat Chronic Toxicity (LOAEL) 0.346 Numeric (log mg/kg_bw/day) Toxicity Hepatotoxicity No Categorical (Yes/No) Toxicity Skin Sensitisation No Categorical (Yes/No) Toxicity T.Pyriformis toxicity 0.332 Numeric (log ug/L) Toxicity Minnow toxicity 2.763 Numeric (log mM)
- 49. Uses : Reduction of BP of Hypertension, reduce bleeding and the treatment of congestive failure. ADV effects : Not available Uses : Profound hypotension.
- 50. Hydralazine Hydrochloride 1-hydrazinophthalazine monohydrochloride Hydralazine may interfere with calcium transport in vascular smooth muscle by an unknown mechanism to relax arteriolar smooth muscle and lower blood pressure. The interference with calcium transport may be by preventing influx of calcium into cells, preventing calcium release from intracellular compartments, directly acting on actin and myosin, or a combination of these actions. This decrease in vascular resistance leads to increased heart rate, stroke volume, and cardiac output. Hydralazine also competes with protocollagen prolyl hydroxylase (CPH) for free iron. This competition inhibits CPH mediated hydroxylation of HIF-1α, preventing the degradation of HIF-1α. Induction of HIF-1α and VEGF promote proliferation of endothelial cells and angiogenesis.
- 51. Membrane primary amine oxidase Hydralazine may interfere with calcium transport in vascular smooth muscle by an unknown mechanism to relax arteriolar smooth muscle and lower blood pressure. The interference with calcium transport may be by preventing influx of calcium into cells, preventing calcium release from intracellular compartments, directly acting on actin and myosin, or a combination of these actions. This decrease in vascular resistance leads to increased heart rate, stroke volume, and cardiac output. Hydralazine also competes with protocollagen prolyl hydroxylase (CPH) for free iron. This competition inhibits CPH mediated hydroxylation of HIF-1α, preventing the degradation of HIF-1α.12 Induction of HIF-1α and VEGF promote proliferation of endothelial cells and angiogenesis.
- 52. Water solubility -1.605 Numeric (log mol/L) Absorption Caco2 permeability 1.394 Numeric (log Papp in 10-6 cm/s) Absorption Intestinal absorption (human) 64.349 Numeric (% Absorbed) Absorption Skin Permeability -2.74 Numeric (log Kp) Absorption P-glycoprotein substrate No Categorical (Yes/No) Absorption P-glycoprotein I inhibitor No Categorical (Yes/No) Absorption P-glycoprotein II inhibitor No Categorical (Yes/No) Distribution VDss (human) -0.224 Numeric (log L/kg) Distribution Fraction unbound (human) 0.313 Numeric (Fu) Distribution BBB permeability -0.233 Numeric (log BB) Distribution CNS permeability -2.454 Numeric (log PS) Metabolism CYP2D6 substrate No Categorical (Yes/No) Metabolism CYP3A4 substrate No Categorical (Yes/No) Metabolism CYP1A2 inhibitior Yes Categorical (Yes/No) Metabolism CYP2C19 inhibitior No Categorical (Yes/No) Metabolism CYP2C9 inhibitior No Categorical (Yes/No) Metabolism CYP2D6 inhibitior No Categorical (Yes/No) Metabolism CYP3A4 inhibitior Yes Categorical (Yes/No) Excretion Total Clearance 0.628 Numeric (log ml/min/kg) Excretion Renal OCT2 substrate No Categorical (Yes/No) Toxicity AMES toxicity Yes Categorical (Yes/No) Toxicity Max. tolerated dose (human) 0.916 Numeric (log mg/kg/day) Toxicity hERG I inhibitor No Categorical (Yes/No) Toxicity hERG II inhibitor No Categorical (Yes/No)
- 53. Uses : Treatment of essential Hypertension. Adv.effects : Faintness, dizziness or weakness. Potassium Channel opener Minoxidil 2,4 –diamino -6-Piperidino pyrimidine -3-oxide Minoxidil is thought to promote the survival of human dermal papillary cells (DPCs) or hair cells by activating both extracellular signal-regulated kinase (ERK) and Akt and by preventing cell death by increasing the ratio of BCl-2/Bax. Minoxidil may stimulate the growth of human hairs by prolonging anagen through these proliferative and anti-apoptotic effects on DPCs. Minoxidil, when used as a vasodilator, acts by opening adenosine triphosphate-sensitive potassium channels in vascular smooth muscle cells. This vasodilation may also improve the viability of hair cells or hair follicles.
- 54. ATP-sensitive inward rectifier potassium channel 1 Minoxidil is thought to promote the survival of human dermal papillary cells (DPCs) or hair cells by activating both extracellular signal-regulated kinase (ERK) and Akt and by preventing cell death by increasing the ratio of BCl-2/Bax. Minoxidil may stimulate the growth of human hairs by prolonging anagen through these proliferative and anti-apoptotic effects on DPCs. Minoxidil, when used as a vasodilator, acts by opening adenosine triphosphate-sensitive potassium channels in vascular smooth muscle cells. This vasodilation may also improve the viability of hair cells or hair follicles.
- 55. Water solubility -2.17 Numeric (log mol/L) Absorption Caco2 permeability -0.064 Numeric (log Papp in 10-6 cm/s) Absorption Intestinal absorption (human) 77.252 Numeric (% Absorbed) Absorption Skin Permeability -2.95 Numeric (log Kp) Absorption P-glycoprotein substrate No Categorical (Yes/No) Absorption P-glycoprotein I inhibitor No Categorical (Yes/No) Absorption P-glycoprotein II inhibitor No Categorical (Yes/No) DistributionVDss (human) 0.105 Numeric (log L/kg) DistributionFraction unbound (human) 0.866 Numeric (Fu) DistributionBBB permeability -0.331 Numeric (log BB) DistributionCNS permeability -3.058 Numeric (log PS) Metabolism CYP2D6 substrate No Categorical (Yes/No) Metabolism CYP3A4 substrate No Categorical (Yes/No) Metabolism CYP1A2 inhibitior No Categorical (Yes/No) Metabolism CYP2C19 inhibitior No Categorical (Yes/No) Metabolism CYP2C9 inhibitior No Categorical (Yes/No) Metabolism CYP2D6 inhibitior No Categorical (Yes/No) Metabolism CYP3A4 inhibitior No Categorical (Yes/No) Excretion Total Clearance 0.759 Numeric (log ml/min/kg) Excretion Renal OCT2 substrate No Categorical (Yes/No) Toxicity AMES toxicity No Categorical (Yes/No) Toxicity Max. tolerated dose (human) 0.022 Numeric (log mg/kg/day) Toxicity hERG I inhibitor No Categorical (Yes/No) Toxicity hERG II inhibitor No Categorical (Yes/No) Toxicity Oral Rat Acute Toxicity (LD50) 2.088 Numeric (mol/kg) Toxicity Oral Rat Chronic Toxicity (LOAEL) 0.69 Numeric (log mg/kg_bw/day) Toxicity Hepatotoxicity Yes Categorical (Yes/No) Toxicity Skin Sensitisation No Categorical (Yes/No) Toxicity T.Pyriformis toxicity 0.15 Numeric (log ug/L) Toxicity Minnow toxicity 2.903 Numeric (log mM)
- 56. Uses : Treatment of severe hypertension and in the topical treatment of androgenic alopecia. Adv.effects : Sudden weight gain, rapid –heart beat, faintness or dizziness. Diazoxide : 7-chloro -3- methyl -2H – 1,2,4 benzothiadiazine -1,1 dioxide. Diazoxide is a non diuretic benzothiadiazine indicated for the management of hypoglycemia in patients who produce an excess of insulin caused by a variety of conditions. Diazoxide inhibits insulin release from the pancreas, by opening potassium channels in the beta cell membrane. Diazoxide is chemically related to thiazide diuretics but does not inhibit carbonic anhydrase and does not have chloriuretic or natriuretic activity. It also exhibits hypotensive activity by reducing arteriolar smooth muscle and vascular resistance
- 57. ATP-sensitive inward rectifier potassium channel 1 Diazoxide inhibits insulin release from the pancreas, by opening potassium channels in the beta cell membrane. Diazoxide is chemically related to thiazide diuretics but does not inhibit carbonic anhydrase and does not have chloriuretic or natriuretic activity. It also exhibits hypotensive activity by reducing arteriolar smooth muscle and vascular resistance. Carbonic anhydrase GI absorption High BBB permeant Yes P-gp substrate No CYP1A2 inhibitor Yes CYP2C19 inhibitor No CYP2C9 inhibitor No CYP2D6 inhibitor No CYP3A4 inhibitor No Log Kp (skin permeation) -6.86 cm/s Uses : Treat Hypertensive emergencies
- 58. Adrenergic neuron blocking agents
- 59. 1.Depleting the stores of the neurotransmitter. 2.Reducing the number of impulses travelling the symapthetic nerves. 3. Anatgonzing the actions of the neurotransmitter on the effector cells. 4. Inhibiting neurotransmitter release. Guanethidine monosulphate 2-hexahydro-1-2H-azocinylethyl guanidine sulfate
- 60. Guanethidine acts at the sympathetic neuro effector junction by inhibiting or interfering with the release and/or distribution of norepinephrine (NE), rather than acting at the effector cell by inhibiting the association of norepinephrine with its receptors. It is taken up by norepinephrine transporters to be concentrated within the transmitter vesicles in place of NE, leading to gradual depletion of NE stores in the nerve endings. Guanethidine at the nerve terminal blocks the release of noradrenaline in response to an action potential. In contrast to ganglionic blocking agents, Guanethidine suppresses equally the responses mediated by alpha-and beta-adrenergic receptors but does not produce parasympathetic blockade. Since sympathetic blockade results in modest decreases in peripheral resistance and cardiac output, Guanethidine lowers blood pressure in the supine position. It further reduces blood pressure by decreasing the degree of vasoconstriction that normally results from reflex sympathetic nervous activity upon assumption of the upright posture, thus reducing venous return and cardiac output more. Sodium-dependent noradrenaline transporter
- 61. GI absorption High BBB permeant No P-gp substrate No CYP1A2 inhibitor No CYP2C19 inhibitor No CYP2C9 inhibitor No CYP2D6 inhibitor No CYP3A4 inhibitor No Log Kp (skin permeation) -7.18 cm/s Uses : Guanethidine is used in the management of moderate to severe hypertension. Adv.effects : Dizziness, waekness and post –exercise hypotesnion.
- 62. Reserpine Serpasil An alkaloid found in the roots of Rauwolfia serpentina and R. vomitoria. Reserpine inhibits the uptake of norepinephrine into storage vesicles resulting in depletion of catecholamines and serotonin from central and peripheral axon terminals. It has been used as an antihypertensive and an antipsychotic as well as a research tool, but its adverse effects limit its clinical use. Reserpine's mechanism of action is through inhibition of the ATP/Mg2+ pump responsible for the sequestering of neurotransmitters into storage vesicles located in the presynaptic neuron. The neurotransmitters that are not sequestered in the storage vesicle are readily metabolized by monoamine oxidase (MAO) causing a reduction in catecholamines.
- 63. Reserpine's mechanism of action is through inhibition of the ATP/Mg2+ pump responsible for the sequestering of neurotransmitters into storage vesicles located in the presynaptic neuron. The neurotransmitters that are not sequestered in the storage vesicle are readily metabolized by monoamine oxidase (MAO) causing a reduction in catecholamines. Uses : Mild to moderate hypertension Synaptic vascular amine transporter