Drugs used in endocrine system updated.pptx
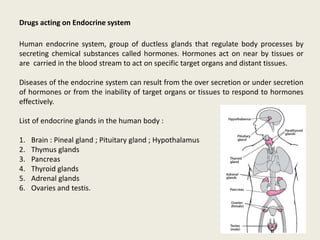
- 1. Drugs acting on Endocrine system Human endocrine system, group of ductless glands that regulate body processes by secreting chemical substances called hormones. Hormones act on near by tissues or are carried in the blood stream to act on specific target organs and distant tissues. Diseases of the endocrine system can result from the over secretion or under secretion of hormones or from the inability of target organs or tissues to respond to hormones effectively. List of endocrine glands in the human body : 1. Brain : Pineal gland ; Pituitary gland ; Hypothalamus 2. Thymus glands 3. Pancreas 4. Thyroid glands 5. Adrenal glands 6. Ovaries and testis.
- 3. Steroid is a class of natural or synthetic organic compounds characterized by a molecular structure of 17 carbon atoms arranged of four rings. Steroids are important in biology, chemistry and medicine. The steroid group includes all the sex hormones, adrenal cortical hormones, bile acids and sterol of vertebrates. Among the synthetic steroids of therapeutic value are a large number of different categories of steroids are frequently distinguished from each other by names that relate to their biological source. Ex : Phytosterols, adrenal steroids and bile acids, androgens and cardiotonic steroids Steroids vary from one another in the nature of the attached groups, the position of the groups and the configuration of the steroid nucleus.
- 4. Steroid numbering system, nomenclature and sterochemistry Gonane : This parent structure, named gonane may be modified in a practically unlimited number of ways by removal, replacement or addition of a few atoms a time. The steroids nucleus is a three dimensional structure and atoms or groups are attached to it by spatially directed bonds. For example, androstane, common to a number of natural and synthetic steroids exists in two forms in which the A/B ring fusions called cis and trans respectively.
- 5. In the cis isomer, bonds to the methyl group, and to the hydrogen atom, H –atom on both the sides they are Beta configuration. Where as the trans isomer, The methyl group projects up and the hydrogen projects down. Steroid structures are represented as plane projection diagrams such as 4 and 5, which correspond 2 and 3 respectively. Stereochemistry of steroids The stereochemistry of the rings markedly affects biological activity of a given class of steroids. There are 6 asymmetric carbon atoms 5,8,9,10,13,14 in the nucleus. There are 64 optically active forms are possible.
- 6. It consist of two conformations – Chair form and boat form. Chair conformation is more stable than boat conformation due to less angle strain. Hence all cyclo hexane rings exist in chair form. H-atom on the β side of the molecule are denoted as solid lines and the α side is denoted by dotted lines. Stereoisomerism based on : 1. the way in which the rings are fused together. 2. The way in which configuration of substituent groups, particularly those at C3 and C17. A/B Fusion : Fusion of rings A and B may either trans or cis to give 2 isomeric C27 hydrocarbons. B/C Fusion : Fusion of the rings B and C was trans. The C10 angular methyl group must be trans to be C9 hydrogen atom. C/D Fusion : The complete X-ray crystallographic analysis of cholesteryl iodide reveals that the ring union is trans in the sterols, bile acids and related steroids.
- 7. 1. Above the plane of the nucleus – β configuration. 2. Below the plane of the nucleus – α configuration. 3. Configuration of substituents is unknown – wavy line. 4. If some carbon atoms are missing in steroid nucleus, the numbering of the remainder will not change Nomenclature
- 10. Cholane Cholestane 5. If side chain doesnot contain methylene group, this is indicated by the prefix “ Nor “ proceeded by the no : of carbon atom that has disappeared. Ex : 23- nor 5α/β cholane
- 11. 23 – nor -5α/β cholane. If the steroid nucleus doesnot contain angular methyl group, this is indicated by the prefix “ Nor” proceeded by the number that designating the methyl group, for example : 10-nor 5 α/β androstane.
- 12. If the ring contraction occur in the steroid nucleus, this is again indicated by the prefix “ nor” proceeded by a capital letter indicting ring affected. For example : A – nor 5α/β androstane A –nor 5α/β androstane If there is enlargement of ring in the steroid nucleus, indicated by the prefix “ Homo” proceeded by a capital letter indicating ring affected. Ex : β-homo - 5α/β pregnane
- 13. If there is a ring fission or breaking occurs, indicated by the prefix “ Seco” proceeded by a number of position of broken bond. For example : 2,3 Seco -5α/β – androstane – 2,3 dioic acid If steroid nucleus contains 3 membered ring, indicated by prefix “ cyclo” – For example : 3α5α - Cyclocholestane
- 14. 3α5α cyclocholestane Metabolism of steroids Steroids are primarily oxidized by cytochrome P450 oxidase enzymes such as CYP3A4. These reactions allow the cholesterol to be broken up by other enzymes in to bile acids.These acids can be eliminated through bile. The steroid hormones, lacking the side chain of cholesterol and bile acids are hydroxylated at various ring positions or oxidized at the 17 position. Which is conjugated with sulfate or glucuronic acid and excreted in the urine.
- 15. Sex hormones A hormone is a chemical released in to the blood and transported to affect cells in other parts of the body. Hormones regulate many things in the body such as growth and development. Male and female gender development; levels of salts and sugars in the blood : The amount of fluid in the body : Sex hormone is a chemical substance produced by a sex gland which has an effect on the sexual features of an organism. Types of male and female hormones Estrogen 13-methyl-6,7,8,9,11,12,14,15,16,17- decahydrocyclopenta[a]phenanthrene-3,17- diol
- 16. Estrogen is a female sex hormone , It is responsible for the development and regulation of the female reproductive system and secondary sex characteristics. There are three major endogenous estrogens : oestrone, oestradiol and oestriol. Among these oestrone and oestradiol is more prevalent. E-1 –OESTRONE E-2 – ESTRADIOL E-3 - OESTRIOL E-4 – ESTETROL – Produced during pregnancy. MOA : Estradiol is a naturally occurring hormone circulating endogenously in females. It is commercially available in several hormone therapy products for managing conditions associated with reduced estrogen, such as vulvovaginal atrophy and hot flashes. Some available forms of estradiol include oral tablets, injections, vaginal rings, transdermal patches, sprays, gels, and creams
- 17. Estrogen is found in the the breast, uterine, ovarian, skin, prostate, bone, fat, and brain tissues. The main source of estrogen in adult women during the reproductive period of life is the ovarian follicle, which secretes 70 to 500 mcg of estradiol each day. After menopause, however, the majority of endogenous estrogen is produced by transformation of androstenedione (which is secreted by the adrenal cortex) to estrone in the peripheral tissues. Both estrone and its sulphate conjugated form, estrone sulphate, represent the most abundant estrogens found in postmenopausal women. Estradiol, however, is considerably more potent than estrone and estriol at the estrogen receptor (ER). Estradiol workings by binding to subtypes of the estrogen receptor: estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ). It also exerts potent agonism of G Protein-coupled estrogen receptor (GPER), which is recognized an important regulator of this drug's rapid effects. Once the estrogen receptor has bound to its ligand, it enters the nucleus of the target cell, regulating gene transcription and formation of of messenger RNA. This mRNA makes contact with ribosomes producing specific proteins that express the effect of estradiol upon the target cell. Agonism of estrogen receptors increases pro- estrogenic effects, leading to the relief of vasomotor and urogenital symptoms of a postmenopausal or low estradiol state
- 18. SAR : 1. Each oestrogen contains a phenolic A ring with a hydroxyl group at carbon 3 abd a beta – OH or ketone in position17 of ring D. 2. The phenolic A ring is the principal structural feature responsible for selective, high affility binding to both receptors. 3. Ring –A and 3-OH, 17β – OH groups is necessary for activity. 4. Activity depends on mode of action subcutaneous - Estradiol ˃˃ Estrone˃ estriol. 5. Removal of 3-OH group, epimerization of 17 β – OH group, introduction of unsaturation in “ β” ring, expansion of “ D” ring drastically decrease the activity. 6. Compound without steroid nucleus also showed activity.
- 20. Uses : commonly used in the birth control and menopausal hormone therapy Adverse reactions : Breast tenderness, breast enlargement, head ache, nausea, fluid retention and edema. Estradiol 13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol
- 21. MOA : Estradiol acts primarily as an agonist of the estrogen receptor ( ER) , a nuclear steroid hormone receptor. There are two types of the estrogen receptor – ER , ER α and Erβ and estradiol potently binds to and activates both of these receptors. The result of ER activation is a modulation of gene transcription and expression in ER – expressing cells, which is the predominant mechanism by which estradiol mediates its biological effects in the body. Metabolism : Estradiol is also metabolized via hydroxylation in to catechol estrogens. It is specifically metabolized by CYP1A2, CYP3A4 and CYP2C9 via 2- hydroxylation in to 2- hydroxy estradiol. Estradiol is additionally conjugated with an ester in to lipoidal estradiol forms like estradiol palmitate and estradiol stearate. Therapeutic uses : Primarily in hormone therapy for menopausal symptoms as well as transgender hormone replacement therapy.
- 22. oestriol 13-methyl-6,7,8,9,11,12,14,15,16,17- decahydrocylcopenta phenantherene – 3,16,17 – triol. MOA : Estriol is an estrogen, specifically an agonist of the estrogen receptors Erα and Erβ. It is a far less potent estrogen than is estradiol and as such is a relatively weak estrogen.
- 23. Metabolism Estriol is metabolized via glucuronidation and sulfation. Therapeutic uses : It is primarily used in the hormone therapy for menopausal symptoms. Oestrione 3-hydroxy – 13 –methyl- 7,8,9,11,12,14,15,16 – octahydro – cyclopenta phenthrene – 17 –one.
- 24. Estrogens enter the cells of responsive tissues (e.g. female organs, breasts, hypothalamus, pituitary) where they interact with estrogen receptors. Hormone-bound estrogen receptors dimerize, translocate to the nucleus of cells and bind to estrogen response elements (ERE) of genes. Binding to ERE alters the transcription rate of affected genes. Estrogens increase the hepatic synthesis of sex hormone binding globulin (SHBG), thyroid- binding globulin (TBG), and other serum proteins and suppress follicle-stimulating hormone (FSH) release from the anterior pituitary. Estrogen receptor alpha Metabolism : estrone is conjugated in to estrogen conjugates such as estrone sulfate and estrone glucuronide by sulfo transferases and glucuronidases and can also bne hydroxylated by cytochrome P450 enzymes in catechol estrogens such as 2- hydroxy estrone and 4- hydroxy estrone. Therapeutic uses : hormone therapy in menopausal symptoms. Adverse effects – Bloating, breast tenderness or swelling, nausea, leg, cramps.
- 25. Diethyl stilbestrol 4- (4- hydroxy phenyl) hex- 3-en-3yl) Phenol MOA : Estrogens diffuse in to their target cells and interact with a protein receptor, the estrogen receptor. Target cells include the female reproductive tract, the mammary gland, the hypothalamus and the pituitary. The effect of estrogen binding their receptors increases the hepatic synthesis of sex hormones binding globulin (SHBG), thyroid binding globulin (TBG) and suppress follicle – stimulating hormone ( FSH) from the anterior pituitary.
- 26. Metabolism : DES is metabolized mainly by glucuronidation and oxidation with the latter including aromatic hydroxylation of the ethyl side chain and dehydrogenation in to dienestrol. Therapeutic uses : Menopausal hormone therapy for the treatment of vaginal atrophy, hot flashes, gonorrheal vaginitis and breast cancer. Adverse reactions : DES is associated with high rates of side effects including nausea, vomiting, abdominal discomfort, headache, and bloating. Progesterone 17-acetyl-10,13-dimethyl- 1,2,6,7,8,9,11,12,14,15,16,17- dodecahydro cyclopenta – phenanthren- 3-one.
- 27. MOA : Progesterone shares the pharmacological actions of the progestins. Progesterone binds to the progesterone and estrogen receptors. Their target cells are female reproductive tract, the mammary gland and hypothalamus and the pituitary. Progesterone will slow the frequency of release of gonadotropin – releasing hormone from the hypothalamus and blunt the pre –ovulatory LH surge. Progesterone is essential for the development of decidual tissue and is necessary to increase endometrial receptivity for implantation of an embryo. Once the embryo has been implanted, progesterone acts to maintain pregnancy. Progesterone stimulated the growth of mammary alveolar tissue and replaces the smooth uterine muscle and thereby producing the androgenic activity. SAR : 1. Steroidal nucleus is essential for activity. 2. Substitution at 17 alpha with ethynyl, methyl, ethyl reduce activity. 3. Ethindrone more active orally than progesterone 4. Removal of methyl group at C-19 is more active – Ex : Nor ethindrone. Nor ethindrone.
- 28. 5. Unsaturation of B or C ring of 19 – Androstane derivatives increase activity. 6. Acetylation of the 17 –Beta – OH of nor ethindrone long duration of action. Metabolism : Progesterone is metabolized primarily by the liver to pregnane diols and pregnanolones. Uses : Taking progesterone by mouth and applying progesterone gel in to the vaginal tract are effective strategies for treating absence of menstrual periods in the menopausal women. Adv.reactions : Stomach upset, changes in appetite, weight gain, fluid retention and swelling, fatigue, drowsiness, insomnia, allergic skin rashes and fever.
- 29. Nandralone 17-Hydroxy -13 –methyl – 2,6,7,8,9,10,11,12,14,15,16,17 do deca hydro – 1 H – cyclopenta phenanthren – 3-one. MOA : Nandrolone is an androgen receptor agonist. It bound to the receptor complexea and allow it to enter the nucleus and bind directly to specific nucleotide sequence of the chromosomal DNA.
- 30. 1. Some of the structural modification that have been introduced in to the testosterone in an attempt to maximize the anabolic effects and minimize the androgenic. 2. Structural modifications to the A and B- rings of testosterone that increase anabolic activity 3. Substitution at C-17 confers oral or depot activity.
- 31. Metabolism : nandrolone is unusual in that unlike most anabolic steroids. It is a less effective product known as Dihydronandrolone. Therapeutic uses : nandrolone esters are used clinically for people in catabolic states with major burns, cancer and AIDS. Adverse reactions : Side effects of nandrolone esters include masculinization among others. Testosterone 17- hydroxy – 13-methyl – 2,6,7,8,9,10,11,12,14,15,16,17 – dodecahydro – 1H – cyclopenta phenanthren – 3-one.
- 32. MOA : The androgen receptor exists in the cytoplasm bound to the heat shock proteins HSP90, HSP70, and other chaperones. After binding to an androgen, the androgen receptor dissociates from HSP90 and undergoes a conformational change to slow the rate of dissociation from the androgen receptor. The androgen-receptor complex is transported into the nucleus where it binds to DNA and recruits other transcriptional regulators to form a pre-initiation complex and eventually induce expression of specific genes. Testosterone and its active metabolite dihydrotestosterone (DHT) antagonize the androgen receptor to develop masculine sex organs including the prostate, seminal vesicles, penis, and scrotum. Antagonism of the androgen receptor is also responsible for the development of secondary sexual characteristics including facial and body hair, enlargement of the larynx, thickening of the vocal cords, and changes in muscle and fat distribution
- 33. SAR : 1. It lacks the 2- carbon side chain attached to the 17- position and making in to a 19- carbon steroid ( an androstane). 2. 17- alpha methyl group increase the duration of action and improve biovailability. 3. The esterification of 17- beta – OH group increases duration of action and bioavailability. 4. The removal of 19 th carbon led to more anabolic selective molecule and less androgenic activity. Metabolism : Testosterone is metabolized to 17 – keto steroids through two different pathways . The major active metabolites are estradiol and di hydro testosterone. Enzyme: UDP- glucuronosyl transferase 1-10 BioSystem: HUMAN
- 34. Enzyme: UDP-glucuronosyltransferase 1-10 BioSystem: HUMAN Therapeutic uses : For management of acquired hypogonadism, hypogonadism associated with HIV infection. Adverse reactions : Acne, swelling, breast enlargement in males.
- 35. Drugs used in gentile dysfunction Sildenafil 5-(2-ethoxy -5 ( 4-methyl piperazin -1yl –sulfonyl)Phenyl) -1- methyl – 3- propyl – 1,6 dihydro -7H – Pyrazolo ( 4,3 –d) pyrimidine – 7-one.
- 36. MOA : Sildenafil inhibits the cGMP – specific phospho diesterase type 5 which is responsible for the degradation of cGMP in the corpus cavernosum which is on the male gentiles.It increases the gentile blood flow resulting in a relaxation of smooth muscle. This response is mediated by the release of Nitric oxide (NO) from nerve cells and endothelial cells which stimulates the synthesis of cGMP in smooth muscle cells. The inhibition of phosphodiesterase type -5 by sildenafil increases the gentile function by increasing the cGMP. Metabolism Sildenafil appears to be completely metabolized in the liver by 16 metabolism. The metabolism is mediated mainly by cytochrome P450 microsomal isoenzymes 3A4 and 2C9, The major circulating metabolite, N – demethylated metabolite has PDE selectively similar to the parent drug. The N- demethylated metabolite is metabolized to an N- dealkylated and N,N – De ethylated metabolite.
- 37. Human Phospho diesterase Enzyme: Cytochrome P450 1A2 BioSystem: HUMAN EAWAG_RULE_BT0425_PATT ERN1
- 38. N-Hydroxylation of primary arylamine N-Hydroxylation of primary arylamine N-Dealkylation of N- acylurea 1-Naphthoxy Photorearrangement_C2 N-Hydroxylation of primary arylamine
- 39. N-Dealkylation of N- acylurea 1-Naphthoxy Photorearrangement _C2 Hydrolysis of oxime N-Glucuronidation of hydroxylamine
- 40. Therapeutic Uses : Treatment of gentile dys function. It is the standard treatment for the dysfunction including for men with diabetes mellitus. Adverse reactions : Head ache, flushing, indigestion, nasal congestion and impaired vision. Tadalafil 6- (1,3 – benzodioxol – 5yl) -2-methyl - hexa hydro pyrazolo – pyrido – Indole -1.4 dione.
- 41. Metabolism Hydrolysis of cyclic tertiary carboxamide Hydrolysis of cyclic tertiary carboxamide
- 42. Therapeutic uses : Tadalafil is used to treat male gentile function problems. Adverse Reactions : Headache, stomach discomfort or pain. Oral Contraceptives They are also called as Birth control pills, which are safe, reliable option for preventing unwanted pregnancy. Most oral contraceptives contain a combination of 2 types of hormones an estrogen and progestin with different combinations. Some pills contain only progestin named as “ Minipill” 1.Estrogen and progestin prevent eggs from being released from the ovaries. 2.Progestin causes thining of the endometrium which prevents implantation of a fertilized egg. 3. Progestin thickness the mucus in the cervix, preventing sperm from reaching the eggs.
- 43. 11- (4-dimethyl amino) phenyl) -17 – Hydroxy -13 –methyl -17- prop-1-ynyl - 1,2,6,7,8,11,12,14,15,16 –deca hydro cyclopenta (a) Phenanthren – 3-one. MOA : The anti progestational activity of mifepristone results from competitive interaction with progesterone ate progesterone – receptor sites. Based on studies with various oral doses in several anaimal species, the compound inhibits the activity of endogenous or exogenous progesterone. Mifepristone
- 44. Metabolism of Mifepristone 16-Hydroxylation of sterol 2-Hydroxylation of sterol 4-Hydroxylation of sterol N-Dealkylation of mixed tertiary amine
- 45. Therapeutic uses : Mifepristone followed by a prostaglandin analog which is used for medical abortion. Adverse reactions : Nausea, vomiting, dry mouth, tiredness, dizziness, headache, muscle pain Norgestril 13-ethyl-17-ethynyl-17-hydroxy – 1,2,6,7,8,9,10,11,12,14,15,16 – dodeca hydro cyclopenta (a) Phenathren – 3-one.
- 46. Norgestrel (and more specifically the active stereoisomer levonorgestrel) binds to the progesterone and estrogen receptors within the female reproductive tract, the mammary gland, the hypothalamus, and the pituitary. Once bound to the receptor, progestins like levonorgestrel will slow the frequency of release of gonadotropin releasing hormone (GnRH) from the hypothalamus and blunt the pre-ovulatory LH (luteinizing hormone) surge. Loss of the LH surge inhibits ovulation and thereby prevents pregnancy. Human Progesterone Therapeutic uses : Norgesterol is used in combination with ethinyl oestradiol for birth control process.
- 47. Levonorgesterol 13-ethyl -17 ethynyl -17 –hydroxy -1,2,6,7,8,9,10,11,12,14,15,16- dodecahydro cyclopenta phenathren – 3-one. MOA : Binds to the progesterone and estrogen receptors. Target cells include the female reproductive tract, the mammary gland, the hypothalamus and the pituitary. Uses : Levonorgestrel is a hormonal medication which is used in a number of birth control methods. ADVERSE EFFECTS – NAUSEA, BREAST TENDERNESS, HEADACHES.
- 48. Corticosteroids Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex of vertebrates as well as the synthetic analogues of these hormones. Two main classes of corticosteroids, glucocorticoids and mineralocorticoids are involved in a wide range of physiological processes including stress response, immune response and regulation of inflammation, carbohydrate metabolism, protein catabolism, blood electrolyte levels and behavior. Cortisone 17- hydroxy – 17- (2-hydroxy acetyl) – 10,13- dimethyl – 1,2,6,7,8,9,12,14,15,16 – decahydrocylcopenta phenathrene -3,11 –dione.
- 49. MOA : Cortisone suppresses the immune system, thus reducing inflammation and attendant pain and swelling at the site of injury. Uses : Cortisone, a glucortocoid and adrenaline are the main hormones released by the body as a reaction to stress. They elevate blood pressure and prepare the body for a fight or flight response. Adverse reactions : Asthma, hyperglycemic, insulin resistance, diabetes mellitus, osteroporosis, anxiety, depression, amenorrhoea, catracts, cushing syndrome. Hydrocortisone 11,17,21 trihydroxy pregn-4ene -3,20 dione
- 50. MOA : Hydrocortisone binds to the cytosolic glucocorticoid receptor. After binding, it form receptor – ligand complex translocates itself in to the cell nucleus, where it binds to many glucocorticoid response elements. The anti inflammatory actions of corticosteroids are thought to involve lipoproteins, phospholipase A2 inhibitory proteins. Meatbolism : Primarily hepatic CYP3A4 Uses : Adrenocortical insufficieny, adrenogenital syndrome, high blood calcium, Rheumatoid arthritis. Adverse reactions : Mood changes, increased risk of infection and swelling. Prednisolone 11,17,21 –Trihydroxypregna-1,4 diene-3,20 dione.
- 51. MOA : Glucocorticoids such as prednisolone can inhibit leukocyte infiltration at the site of inflammation. It interfere with mediators of inflammatory response and supress humoral immune responses. The anti – inflammatory actions of glucocorticoids are through to involve Phospholipase A2 inhibitory proteins, lipocortins which control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes. Metabolism : Excreted in the urine as either free of gluco conjugate Thera peutic uses : Asthma, Uveitis, pyoderma gangrenosum, rheumatoid arthritis, crohns disease, Bells palsy, systemic lupus. Adverse reactions : Increased appetite, weight gain, nausea and malaise. Betamethasone 9- fluoro -11,17 –dihydroxy-17 – ( 2- hydroxy acetyl) – 10,13,16 – trimethyl – 6,7,8,9,10,11,12,13,14,15,16,17 – dodeca hydro – 3H – cyclopenta phenathren – 3-one.
- 52. MOA : Betamethasone is a glucocorticoid receptor agonist. This leads to changes in genetic espression once this complex binds with GRE. The anti inflammatory actions of the corticosteroids are thought to involve lip[ocortins, phospholipase A2 inhibitory actions through inhibition of arachidonic acid, conntrol the biosynthesis of prostaglandins and leukotrienes. Betamethasone binds to plasma transcortin and it becomes active when it is not bound to transcortin.
- 53. Therapeutic uses : It is used for a number of diseases including rheumatic disorders such as rheumatoid arthritis and systemic lupus erythematosus, skin diseases such as dermatitis and psoriasis, allergic conditions such as asthma and angiodema. Adv.report : Muscle weakness, severe allergic reactions, psychosis. Dexamethasone Dexamethasone is a glucocorticoid available in various modes of administration that is used for the treatment of various inflammatory conditions, including bronchial asthma, as well as endocrine and rheumatic disorders.
- 54. The short term effects of corticosteroids are decreased vasodilation and permeability of capillaries, as well as decreased leukocyte migration to sites of inflammation. Corticosteroids binding to the glucocorticoid receptor mediates changes in gene expression that lead to multiple downstream effects over hours to days. Glucocorticoids inhibit neutrophil apoptosis and demargination; they inhibit phospholipase A2, which decreases the formation of arachidonic acid derivatives; they inhibit NF-Kappa B and other inflammatory transcription factors; they promote anti- inflammatory genes like interleukin-10.3 Lower doses of corticosteroids provide an anti-inflammatory effect, while higher doses are immunosuppressive.3 High doses of glucocorticoids for an extended period bind to the mineralocorticoid receptor, raising sodium levels and decreasing potassium levels.
- 55. Biotransformation of the Dexamethasone 2-Hydroxylation of sterol 4-Hydroxylation of sterol Epoxidation of alkene Oxidation of primary alcohol to aldehyde
- 56. Uses : Rheumatic problems, severe allergies, asthma, COPD, Croup, brain swelling, along with antibiotics in tuberculosis. Thyroid and Antithyroid drugs Thyroid gland produces thyroid hormones. These hormones are essential for the body metabolism, growth and development. Thyroid hormones are derivatives of the amino acid tyrosine bound covalently to iodine. The two principal thyroid hormones are T3 and T4. Thyroid hormones have two major physiological effects – They increase protein synthesis in every body tissue and increase oxygen consumption dependent upon Na+/K+ ATP ase. Certain types of thyroid tumors appear to be more prevalent in certain age groups. Thyroid problems have a strong genetic component and may skip generations. Hypothyroidism in Adults :
- 57. Hypothyroidism is a common condition where the thyroid doesn’t create and release enough thyroid hormone into your bloodstream. This makes your metabolism slow down. Also called underactive thyroid, hypothyroidism can make you feel tired, gain weight and be unable to tolerate cold temperatures. The main treatment for hypothyroidism is hormone replacement therapy. Thyroiditis Thyroiditis is the inflammation of thyroid gland can be divided in to categories of auto immune ( long –term inflammatory disease ; Grave’s disease, Hashimoto’s disease), Riedel’s and miscellanous. More common in women than in men. Hyperthyroidism Hyperthyroidism is a metabolic imbalance that results from thyroid hormone over production. Grave’s disease Toxic goiter is a cause of 80% of hyperthyroidism. Highest incidence between ages of 30 and 40.
- 58. Antithyroid drugs : Anti –thyroid drugs are compounds that interfere with the body’s production of thyroid hormone, thereby reducing symptoms of hyperthyroidism. ATDs were discovered accidentally in the mid – 1940 when thiocyanate compounds used for heart disease were found to cause hypothyroidism. L-Thyroxine 2-Amino-3 (4- (4-hydroxy-3,5-diiodophenoxy) -3,5 – diiodophenyl) Propanoic acid.
- 59. Levothyroxine is a synthetically prepared levo-isomer of the thyroid hormone thyroxine (T4, a tetra-iodinated tyrosine derivative) that acts as a replacement in deficiency syndromes such as hypothyroidism. T4 is the major hormone secreted from the thyroid gland and is chemically identical to the naturally secreted T4: it increases metabolic rate, decreases thyroid-stimulating hormone (TSH) production from the anterior lobe of the pituitary gland, and, in peripheral tissues, is converted to T3. Thyroxine is released from its precursor protein thyroglobulin through proteolysis and secreted into the blood where is it then peripherally deiodinated to form triiodothyronine (T3) which exerts a broad spectrum of stimulatory effects on cell metabolism. T4 and T3 have a relative potency of ~1:4. SAR : 1. The ether oxygen can be replaced iso sterically by sulphur or methylene which also provide an angle of 120̊ C. 2. A phenolic hydroxyl group at 4’ position is important for hydrogen bonding to transport proteins. 3. The 3’ – position ortho to the hydroxyl and away from the other aromatic ring must be substituted by a lipophilic group. 4. The iodine atoms at 3 and 5 positions can be replaced by non –polar groups. 5. The amino acid side chain can be varied, but should be para to the aromatic ring.
- 60. Metabolism The primary pathway of thyroid hormone metabolism is through sequential deiodination. The liver is the main site of T4 deiodination and along with kidneys are responsible for about 80% of circulating T3. Therapeutic uses : Levo thyroxine is used to treat hypothyroidism. Adv.Reactions : Abdominal pain, nausea, anxious ness, confusion, agitation and insomnia.
- 61. L- Thyroinine 2- amino -3 (4 (4- hydroxy phenoxy) phenyl) propanoic acid. Propyl thiouracil 6-propyl-2-sulfanylpyrimidin-4-one.
- 62. MOA: Propylthiouracil binds to thyroid peroxidase and thereby inhibits the conversion of iodide to iodine. Thyroid peroxidase normally converts iodide to iodine (via hydrogen peroxide as a cofactor) and also catalyzes the incorporation of the resulting iodide molecule onto both the 3 and/or 5 positions of the phenol rings of tyrosines found in thyroglobulin. Thyroglobulin is degraded to produce thyroxine (T4) and tri-iodothyronine (T3), which are the main hormones produced by the thyroid gland. Therefore propylthiouracil effectively inhibits the production of new thyroid hormones. Thyroid peroxidase
- 63. Metabolism Hydroxylation of antepenultimate aliphatic secondary carbon Hydroxylation of penultimate aliphatic secondary carbon Hydroxylation of terminal methyl AndFromCyProduct Terminal desaturation
- 64. Uses : Propyl thiouracil is a medication used to treat hyperthyroidism Adverse effects : Itchiness, hairlosing, swelling, vomiting, musclepains, numbness and headache. Methimazole Methyl-3H-Imidazole – 2-thione Methimazole's primary mechanism of action appears to be interference in an early step in thyroid hormone synthesis involving thyroid peroxidase (TPO), however the exact method through which methimazole inhibits this step is unclear. TPO, along with hydrogen peroxide, normally catalyzes the conversion of iodide to iodine and then further catalyzes the incorporation of this iodine onto the 3 and/or 5 positions of the phenol rings of tyrosine residues in thyroglobulin. These thyroglobulin molecules then degrade within thyroid follicular cells to form either thyroxine (T4) or tri-iodothyronine (T3), which are the main hormones produced by the thyroid gland.
- 65. Metabolism Isoflavanone C-ring fission Isoflavanone C-ring fission Primarily Hepatic
- 66. Therapeutic uses : Hyperthyroidism such as in Grave’s disease, a condition that occurs when the thyroid gland begins to produce an excess of thyroid hormone. ***************Completed************