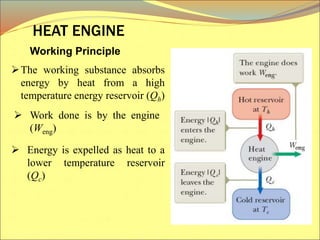
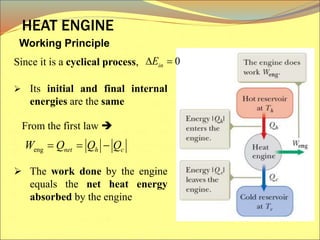
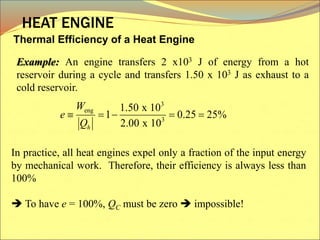
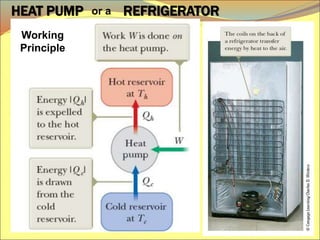
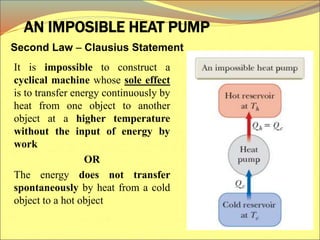
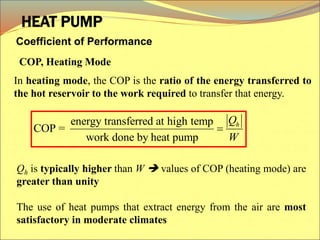
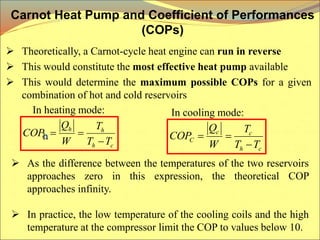
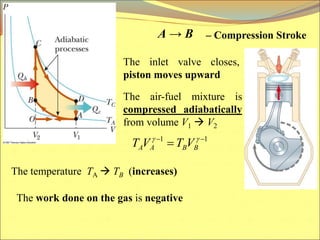
The document describes the working principles of a heat engine. It begins by explaining that a heat engine takes in energy by heat from a high temperature reservoir and expels a fraction of that energy as work during a cyclic process. It then provides more details on the specific processes involved, including absorbing heat, rejecting heat to a lower temperature reservoir, and doing work. The thermal efficiency of a heat engine is also defined.