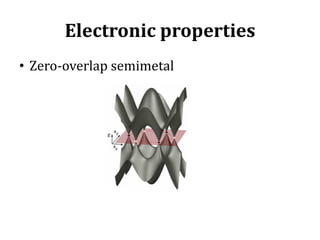
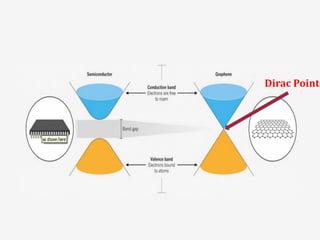
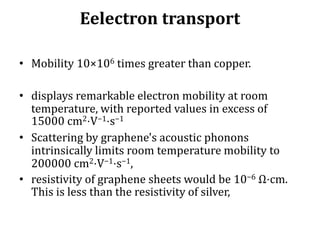
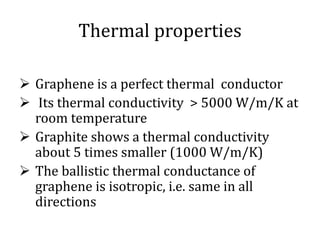
Graphene has potential applications in nanotechnology due to its unique properties at the nanoscale. It is the thinnest material known, with high thermal and electrical conductivity. Early attempts to isolate graphene were unsuccessful, but in 2004 researchers developed a "Scotch tape method" to peel off single-atom thick sheets from graphite, revealing graphene's hexagonal structure under an atomic force microscope. Graphene's electronic properties make it a zero-overlap semimetal with high electron mobility, and its lattice structure results in remarkable thermal conduction properties superior to other materials like diamond. These characteristics could enable new technologies if graphene sheets can be effectively manufactured.




























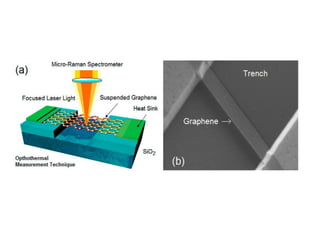
![Measurements of heat conduction
• The
first measurements of
heat conduction in
graphene [35–40]
were carried out at UC
Riverside in 2007
•
optothermal Raman
measurement
technique.](https://image.slidesharecdn.com/e086db49-a311-42cb-9ae5-db720d10aac1-160610192119/85/GRAPHENE-39-320.jpg)