Embed presentation






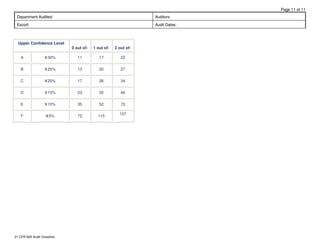
This document is an audit checklist for a quality system audit based on 21 CFR 820 regulations. It contains 11 pages reviewing requirements across multiple quality system elements including design controls, document controls, purchasing controls, process validation, device labeling, non-conforming product handling, corrective and preventive action, and records. The checklist provides sections to review procedures, look for specific items, and record observations. It will be used to audit a department to ensure compliance with FDA quality system regulations.










