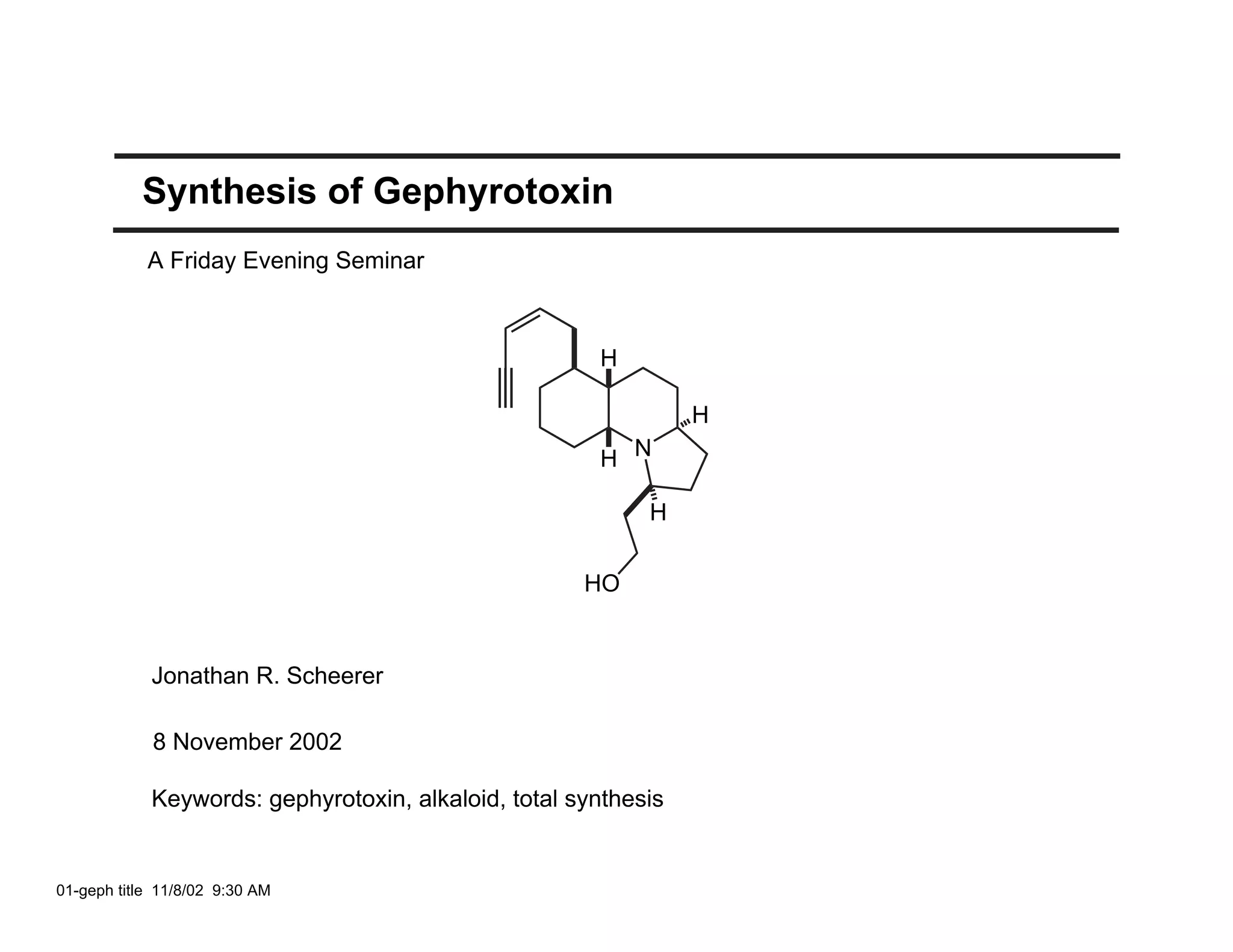
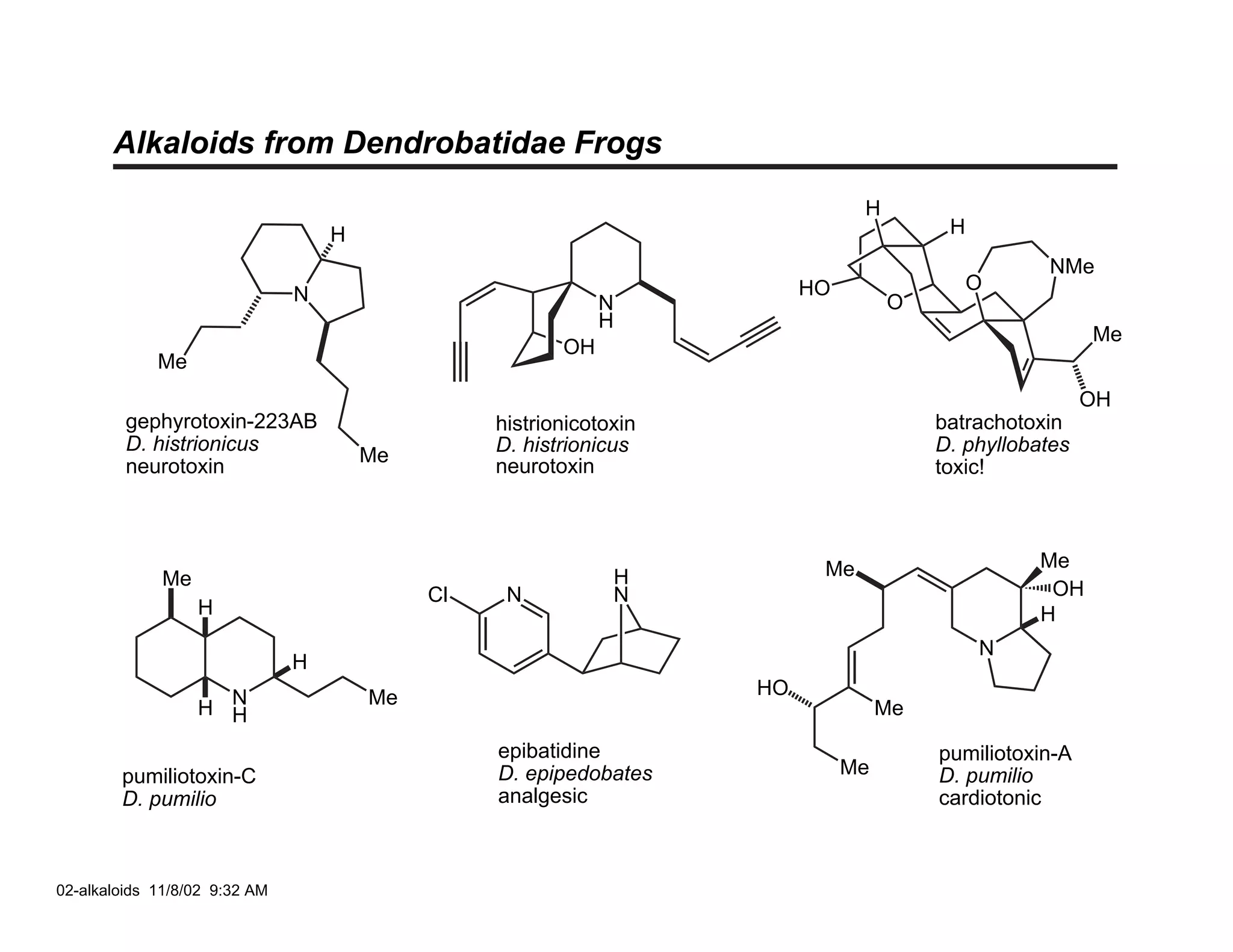
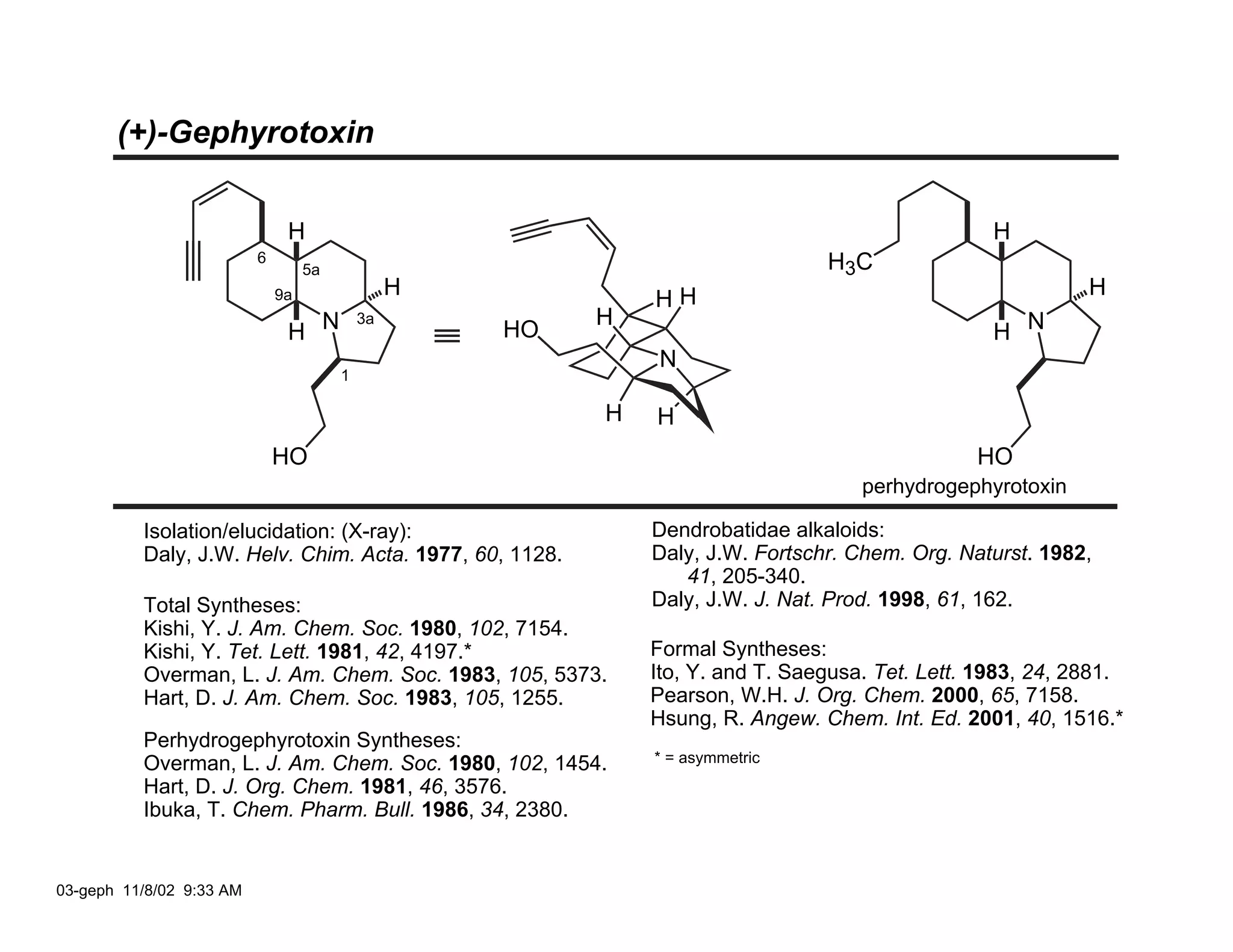
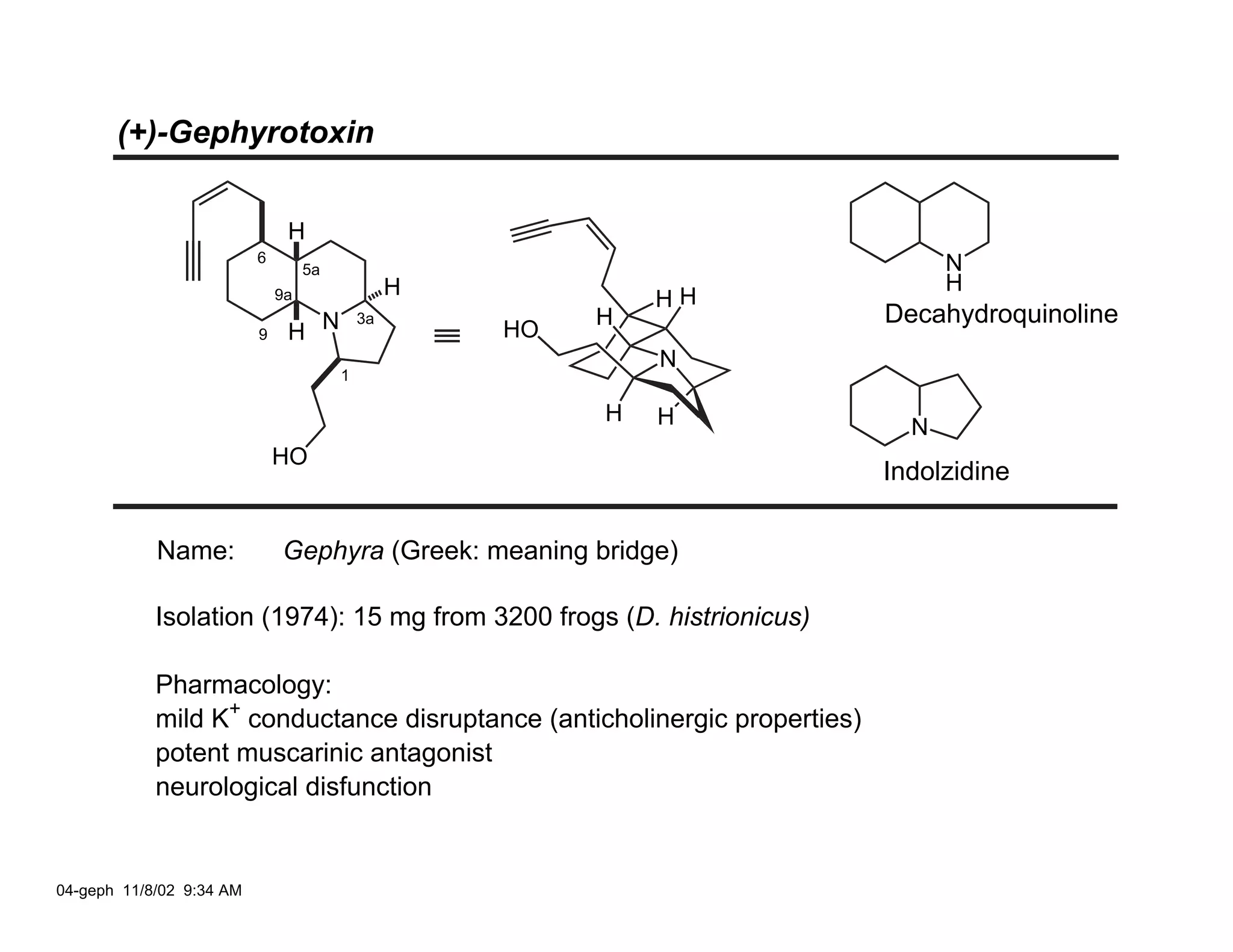
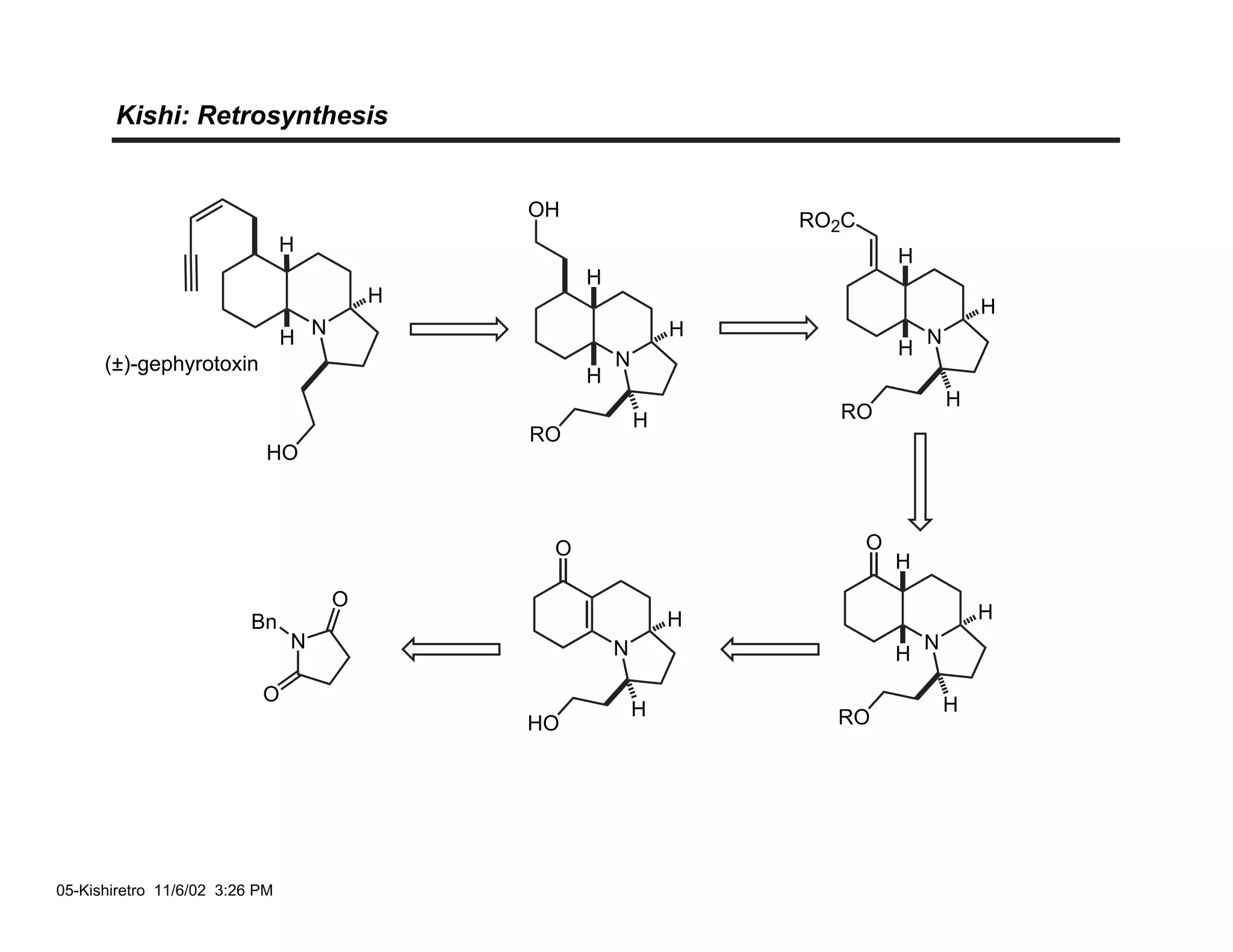
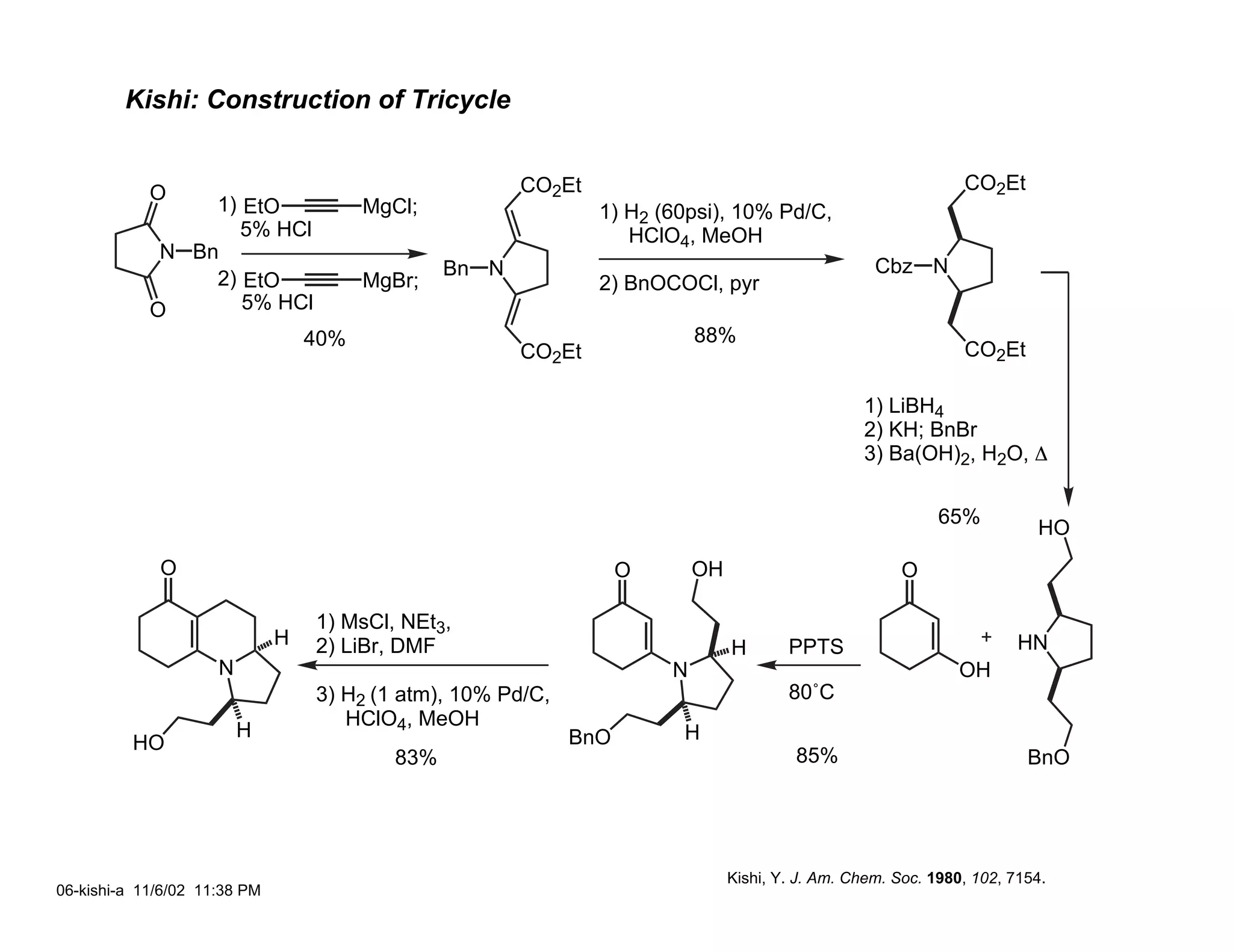
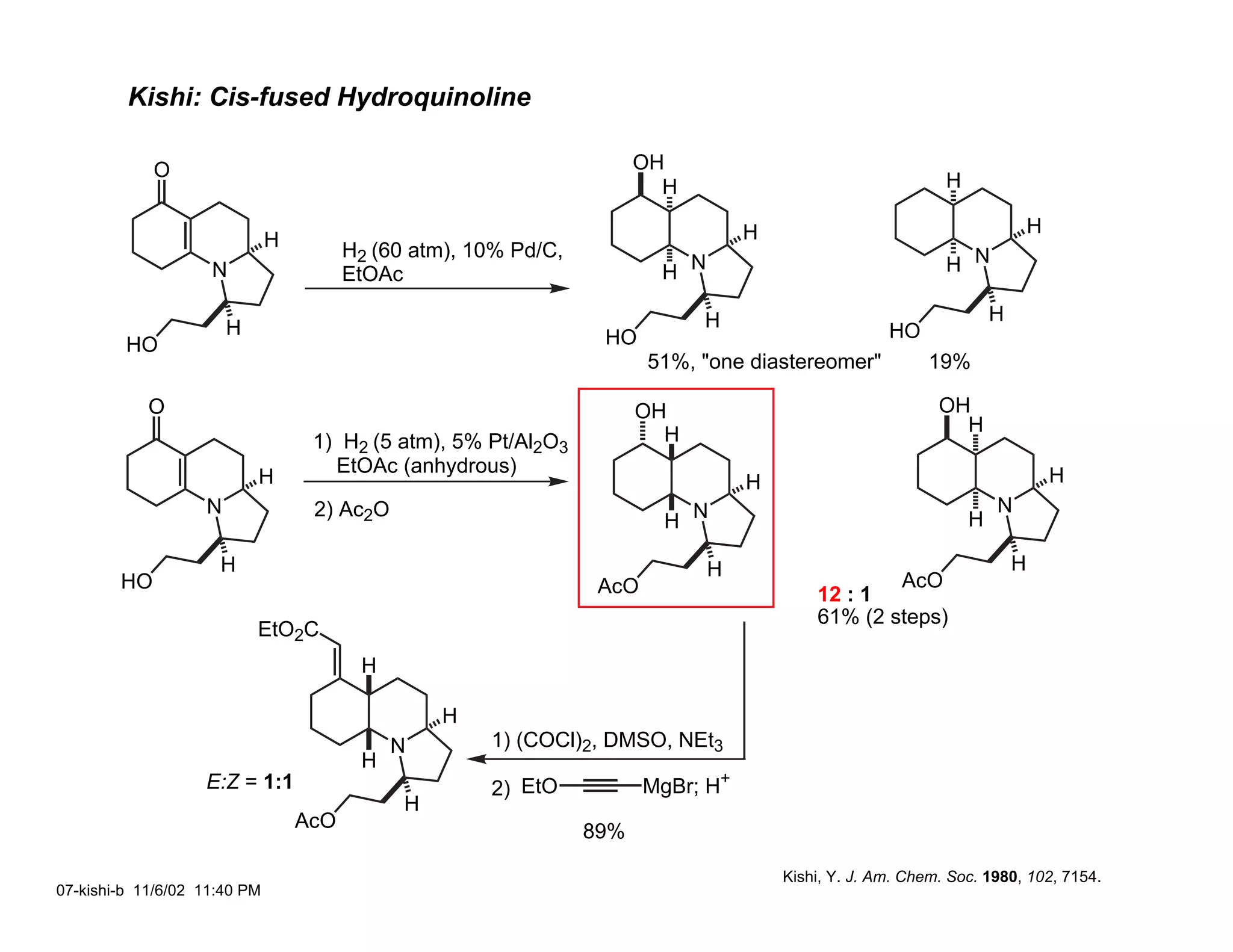
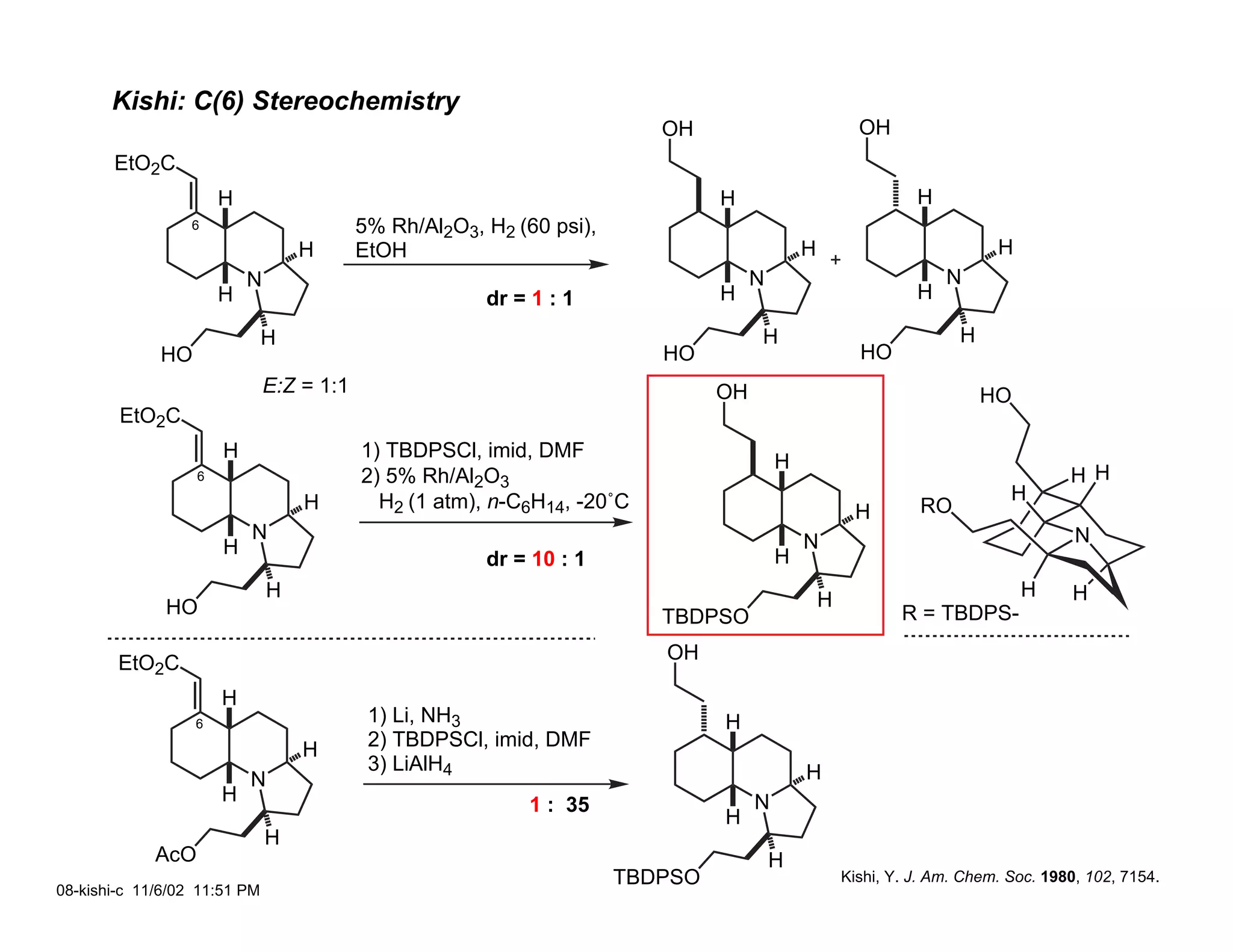
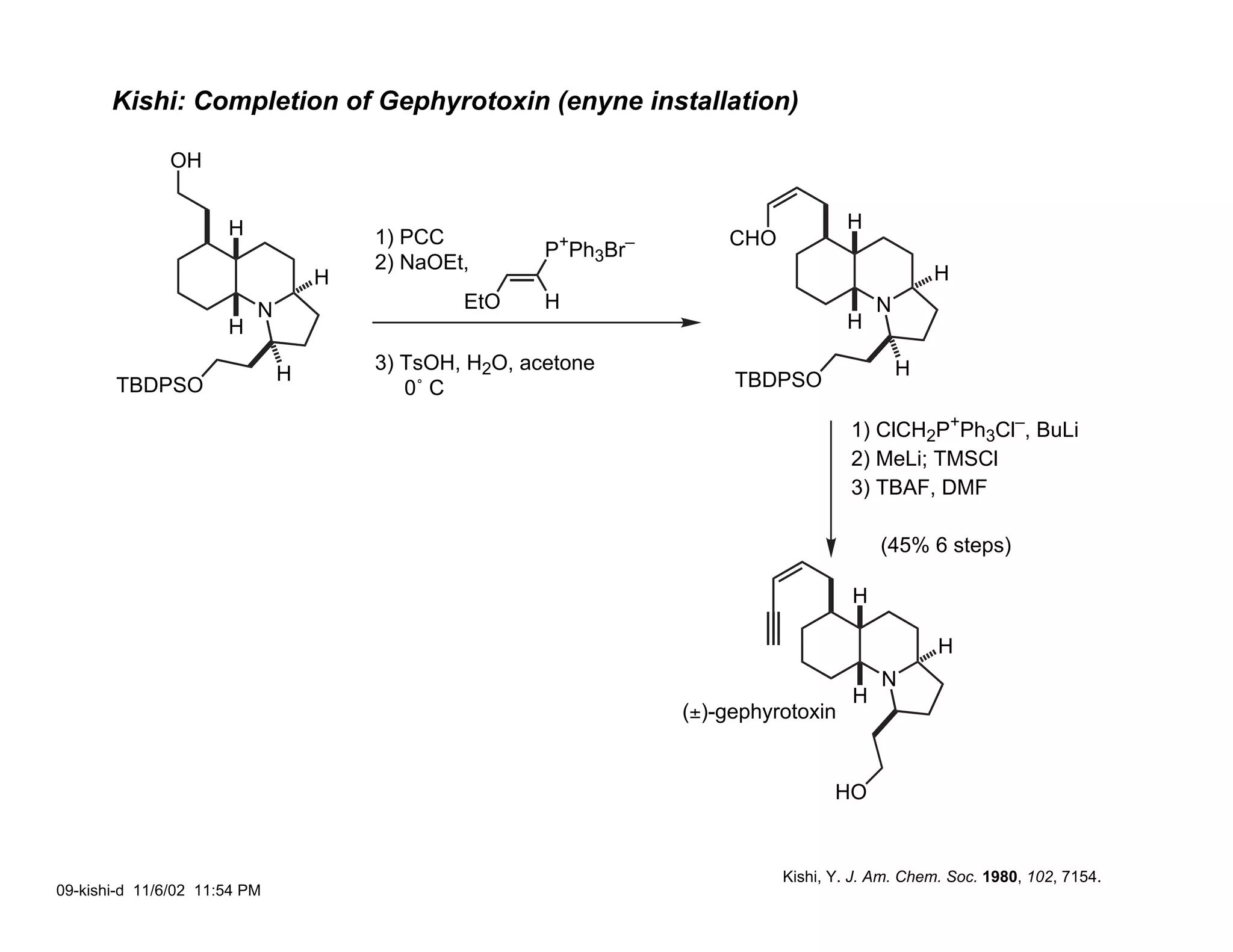
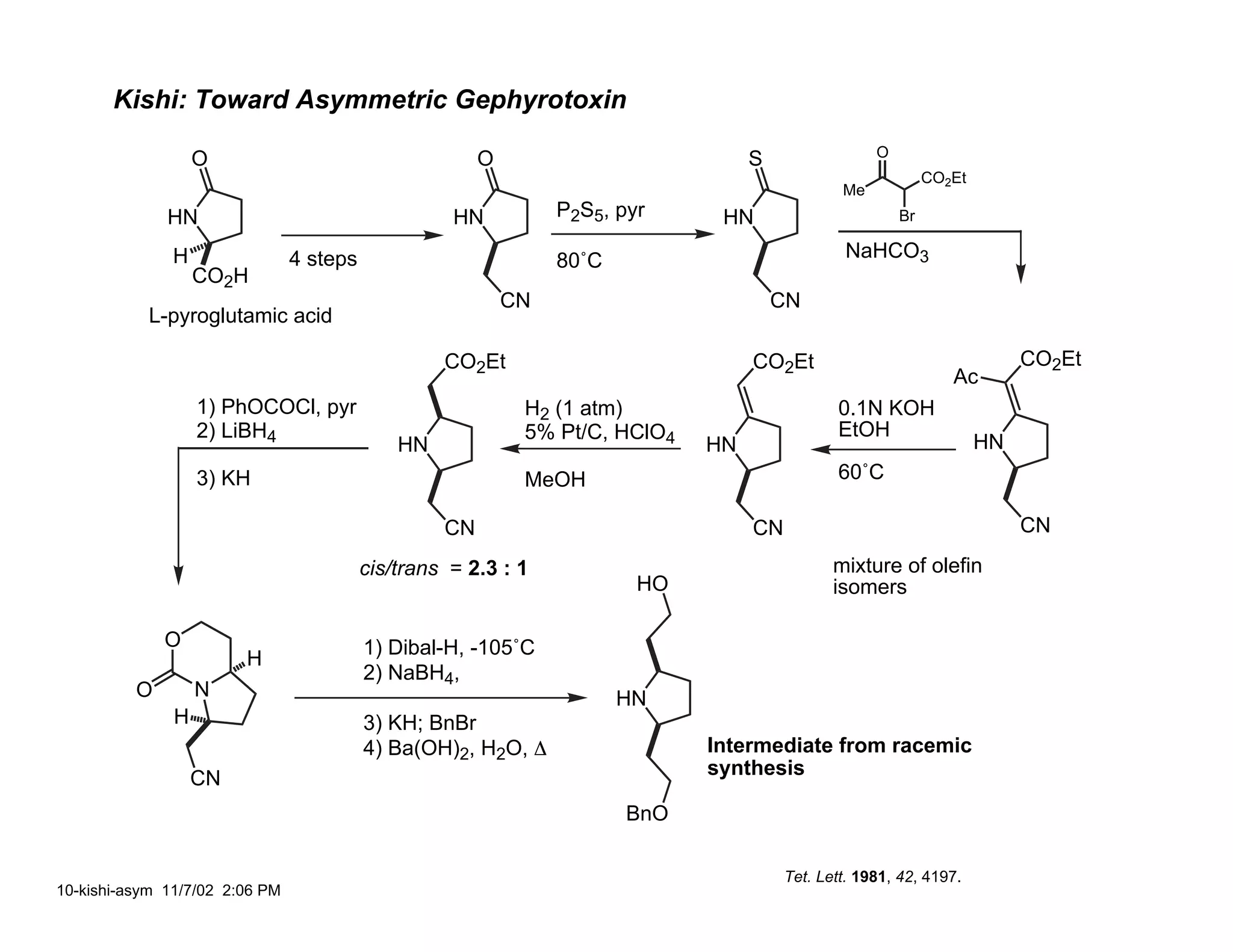
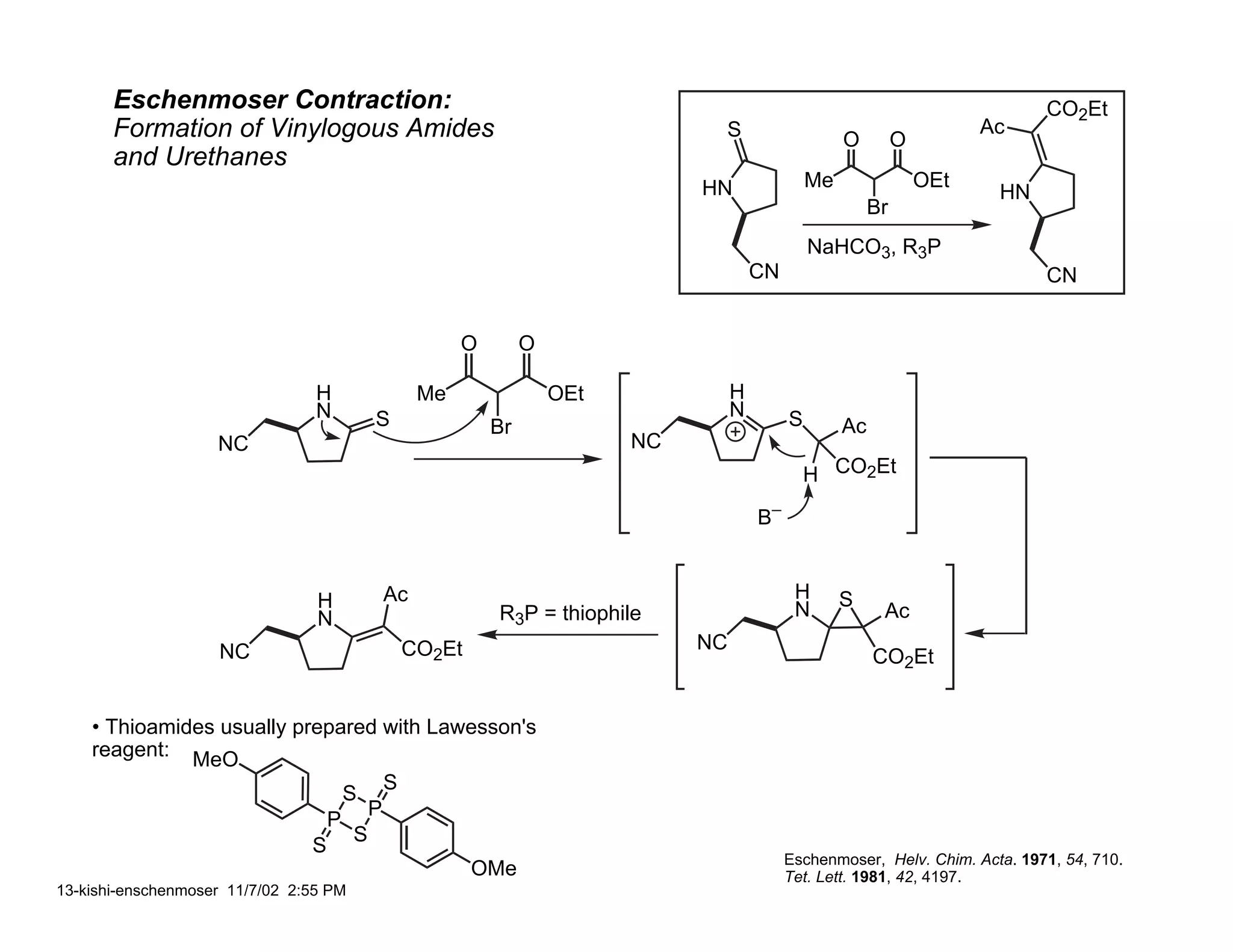
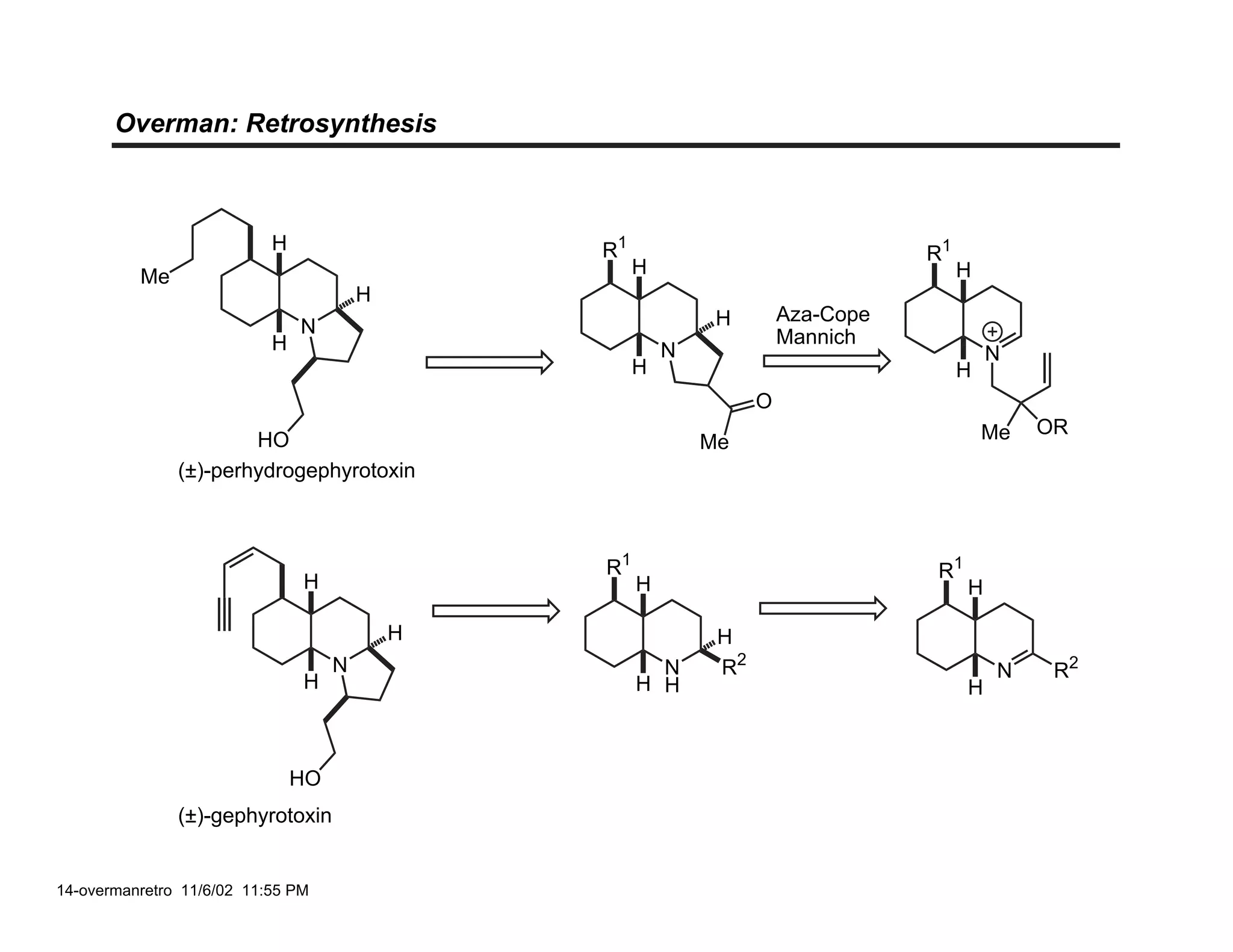
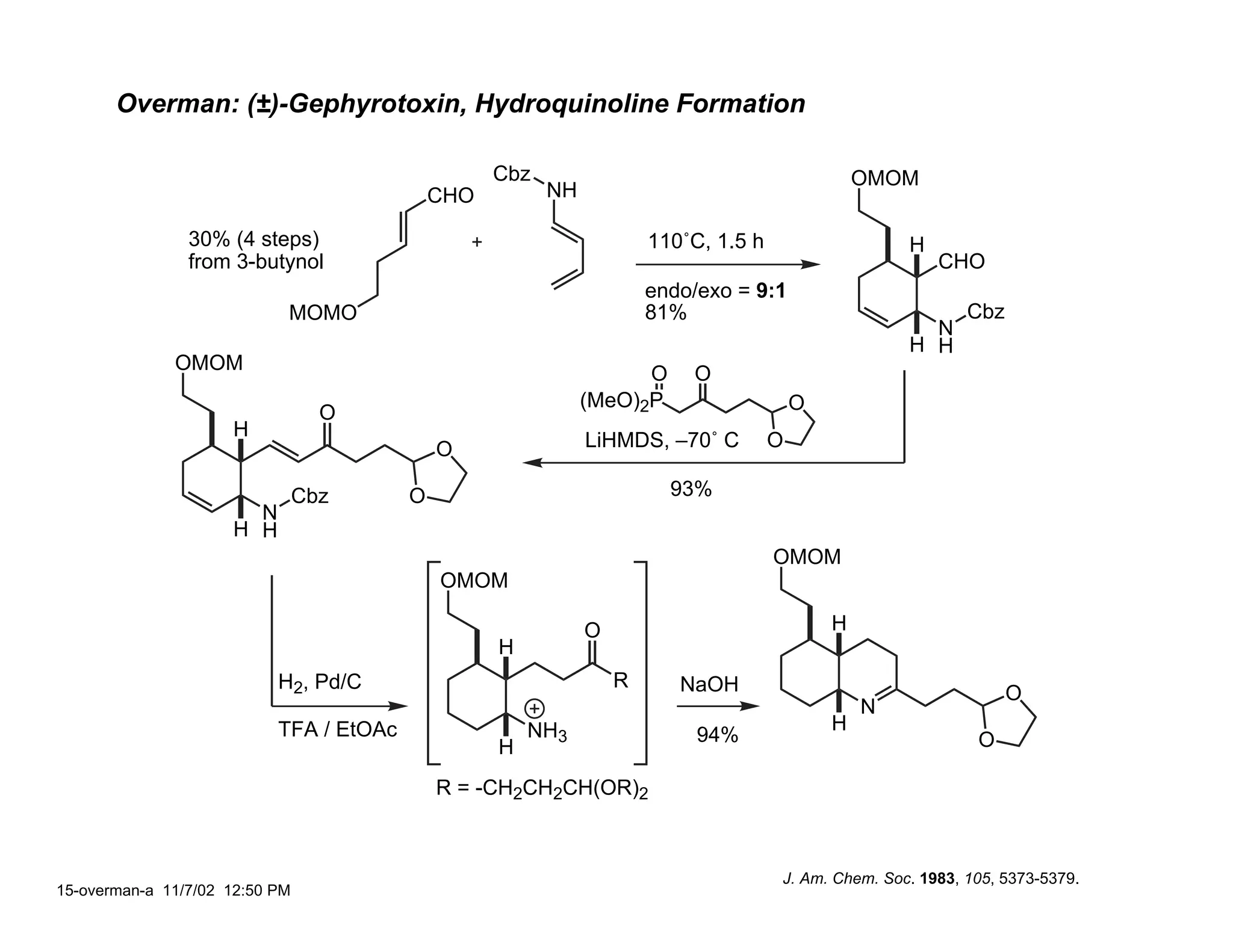
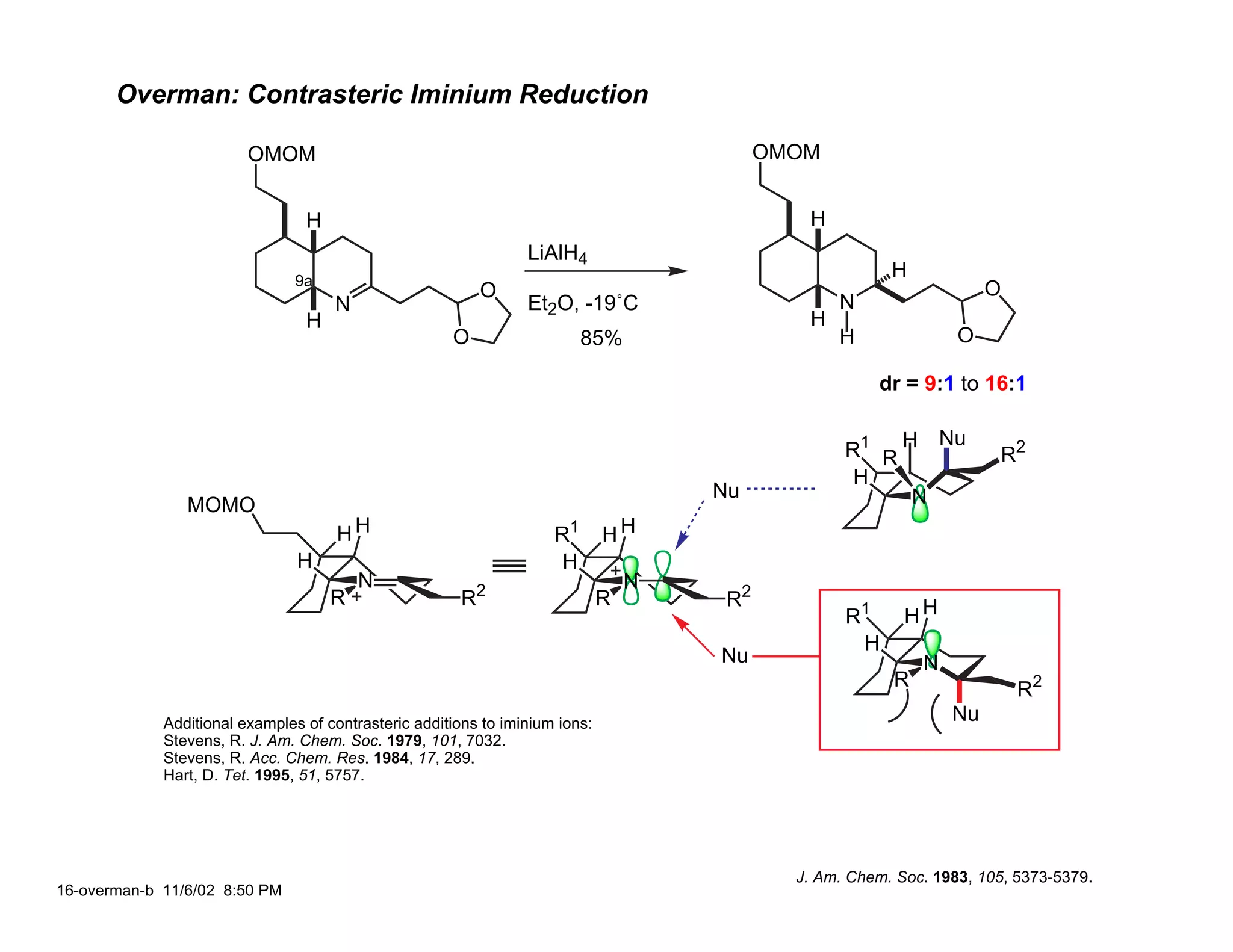
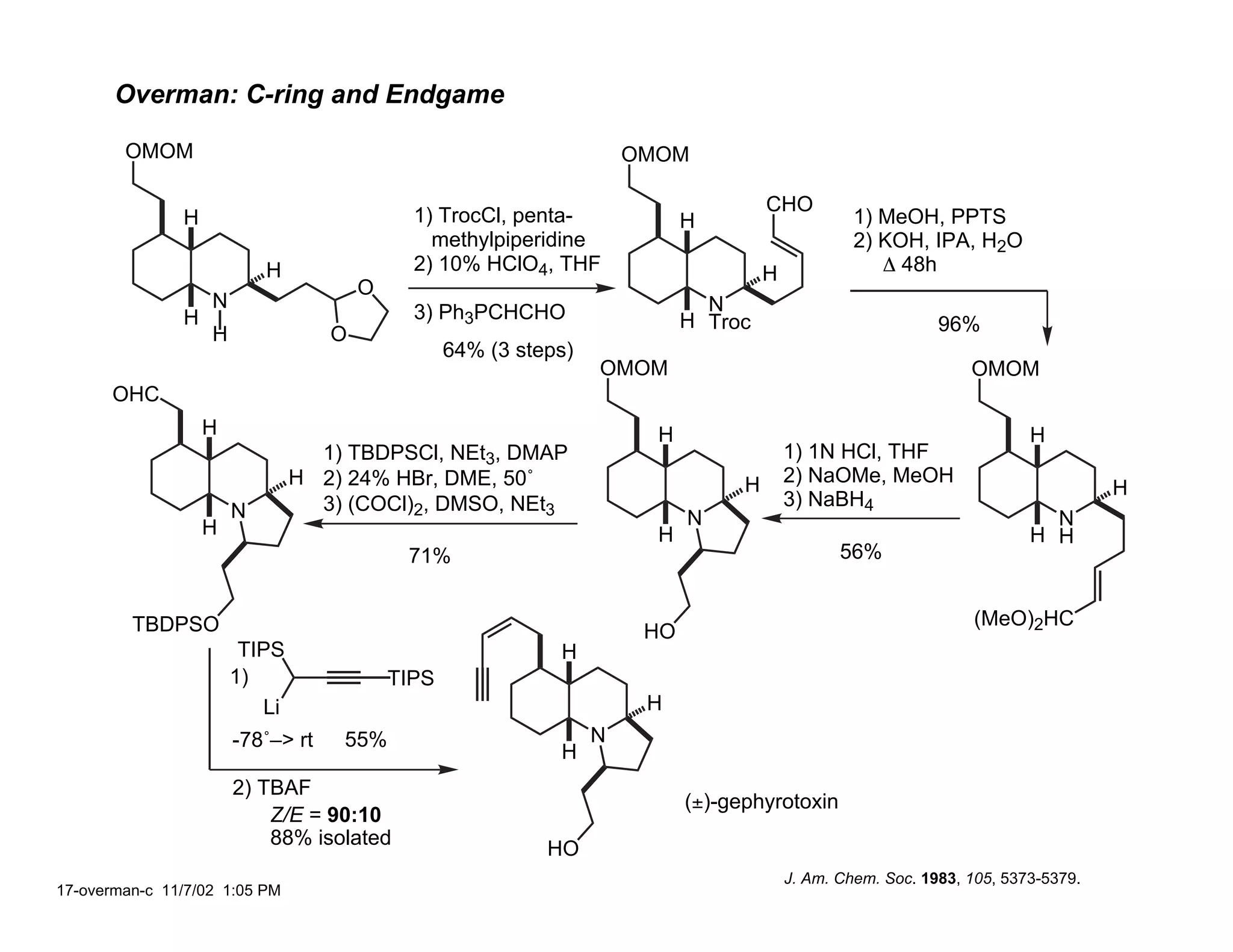
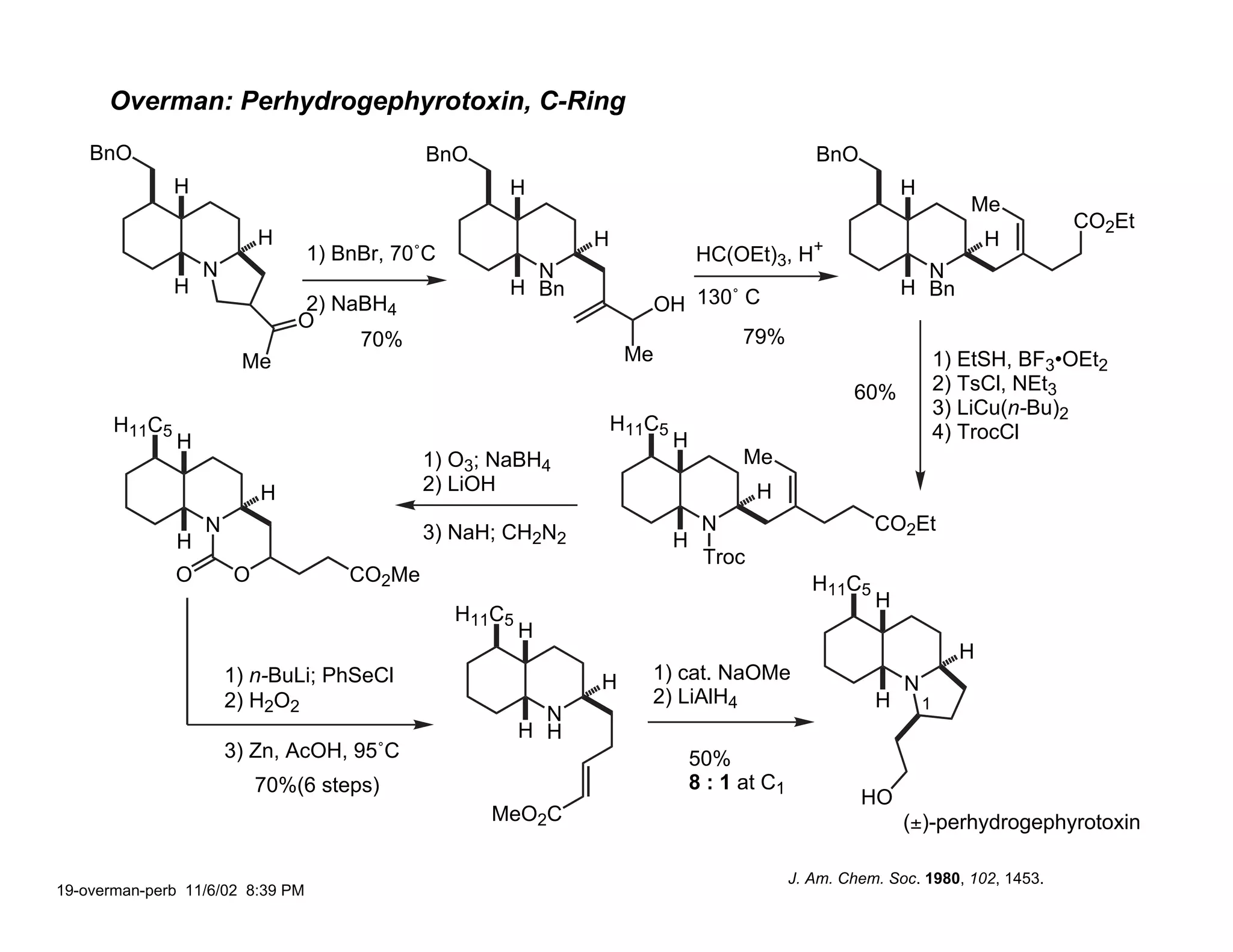
Jonathan R. Scheerer presented on the total synthesis of the alkaloid gephyrotoxin. Gephyrotoxin was first isolated from poison dart frogs and is a potent muscarinic antagonist. Several research groups, including Kishi and Overman, have achieved the total synthesis of both the natural and unnatural enantiomers of gephyrotoxin using retrosynthetic analyses involving aza-Cope rearrangements, Eschenmoser contractions, and stereoselective reductions. The seminar focused on the key steps that these groups used to construct the tricyclic core and install the functionalized enyne portion of the gephyrotoxin molecule.
![Kishi: Absolute Configuration Questioned
HO
H Original stereoconfiguration assigned
by X-ray from HBr salt of gephyrotoxin
H (anomalous scattering of Br–)
HN
N
H
Kishi et al.
12 steps
BnO (+)-gephyrotoxin
HO
Synthetic α
[α]D = +50.0˚
(c =1.0, EtOH)
H Natural α
[α]D = –51.5˚
(c =1.0, EtOH)
H
N Kishi suggests structural reassignment based on
H
optical rotation.
natural (–)-gephyrotoxin (?) Insufficient natural supply to confirm
HO reassignment
Tet. Lett. 1981, 42, 4197.
12-Kishi-asym2 11/7/02 3:16 PM](https://image.slidesharecdn.com/gephyrotoxinevans-120416081537-phpapp02/75/Gephyrotoxin-11-2048.jpg)
![Overman: (±)-Perhydrogephyrotoxin, Hydroquinoline Formation
Cbz
CHO NH BnO
H
CHO 1) Ph3PCHCHO
110˚C, 1.5 h 2) PPTS, MeOH
OBn Cbz
81% N 47%
H H
endo/exo = 10 : 1
BnO BnO
OMe OMe
H 1) 3 equiv H2, Pd/C H
2) NaBH3CN, Me OMe
OMe OMe
OHC Cbz
N N
H H H H
Me OMe 91%
BnO
TsOH, PhH
80˚C R H
H
H H
R Me H
N
H N N H
H
[3,3] O
MeO H Me 79%
Me
OMe 3:2 mix of acyl
diastereomers
J. Am. Chem. Soc. 1980, 102, 1453.
18-overman-pera 11/6/02 11:59 PM](https://image.slidesharecdn.com/gephyrotoxinevans-120416081537-phpapp02/75/Gephyrotoxin-17-2048.jpg)
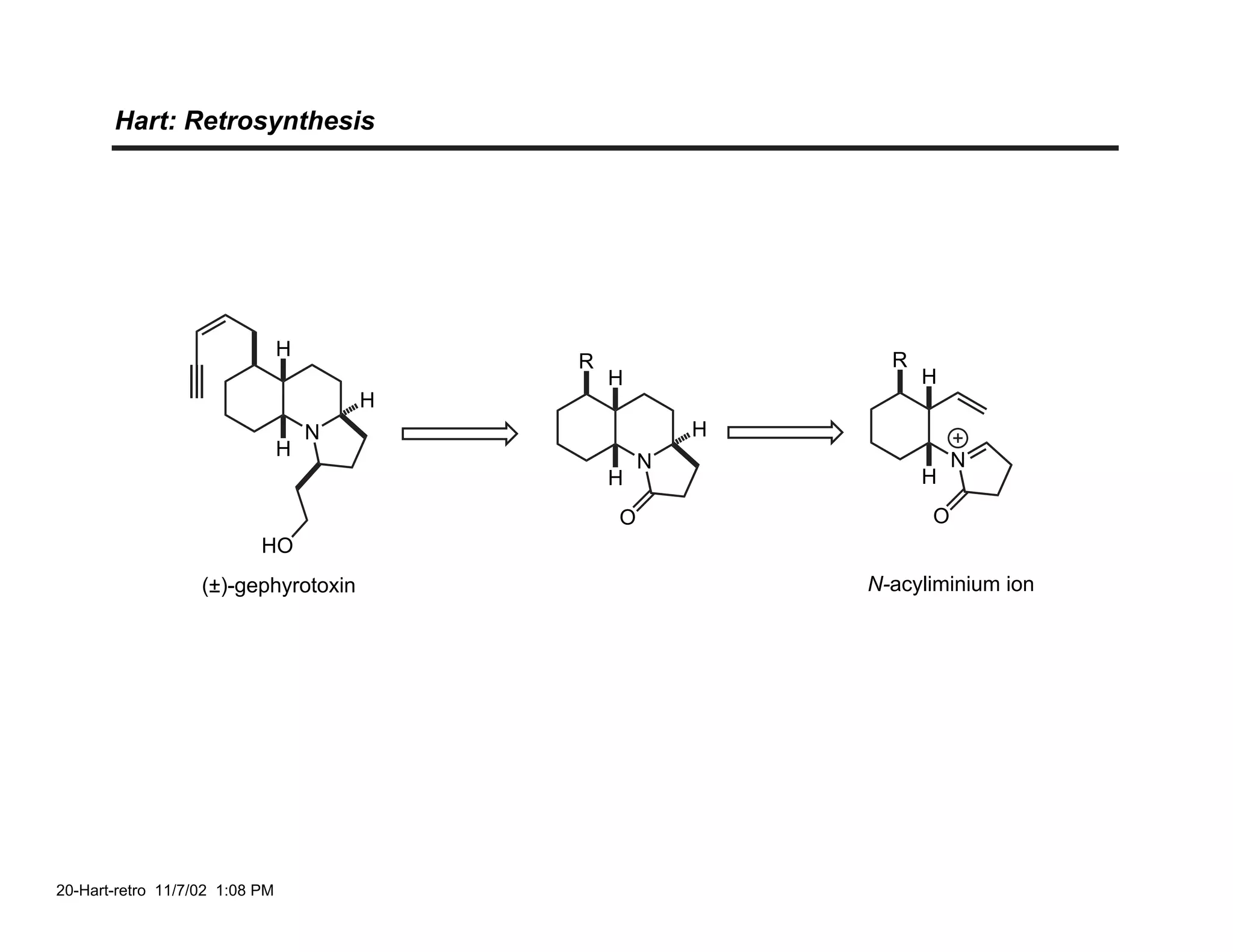
![Hart: N-Acyliminium Ion Cyclization
H
H 1) PPh3, DEAD, succinimide
H
LiAlH4 2) O3; NaBH4 O
H O H
3) Bu3P, o-nitrophenyl- N
O 92% – OH
[H] selenocyanate
Wenkert. et .al. 4) H2O2 O
dr (-70˚C) = 8 : 1 38%
H
Nu O O
Nu O
N HCO2H Dibal-H
H N H N
H N
O H OH 80% O
A(1,3)
Nu = –OCHO
OCHO
H 1) NaOH, MeOH H
OCHO 2) NaH, S2C, imid.
H MeI, 60˚C H
H N
N N
H H 3) Bu3SnH, 110˚C H
O
79% O 65% O
Wenkert. Synth. Commun. 1979, 9, 391.
J. Am. Chem. Soc. 1983, 105, 1255.
21-Hart-a 11/7/02 1:13 PM](https://image.slidesharecdn.com/gephyrotoxinevans-120416081537-phpapp02/75/Gephyrotoxin-20-2048.jpg)
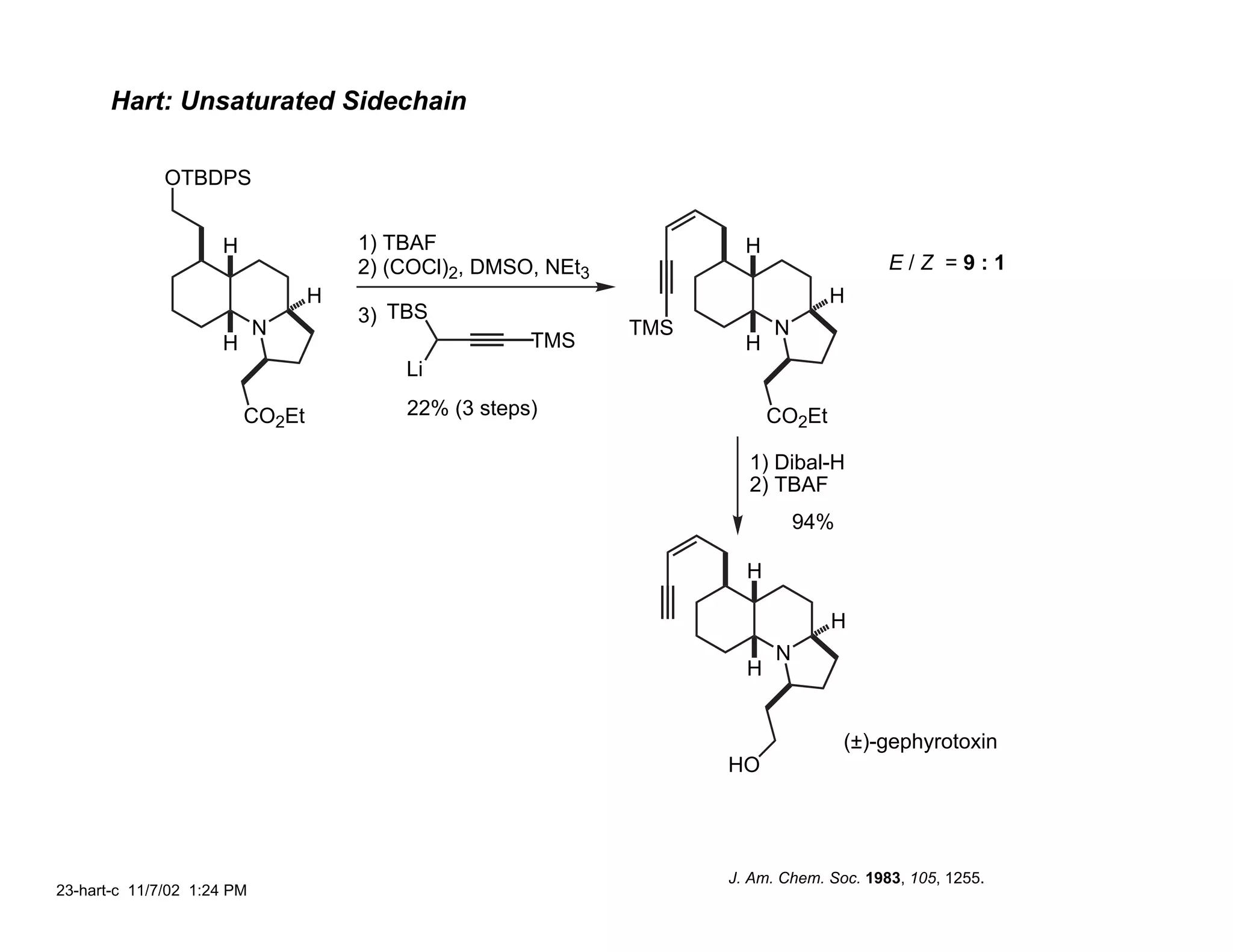
![Hart: C(1) Reduction
OR
H H 1) (sia)2BH;
1) Lawesson's H
2) ethyl bromoacetate; H2O2, NaOH
H Et3N, Ph3P H 2) TBDPSCl, imid. H
N N
H H N
80% 73% H
O
CO2Et
R = H- or TBDPS- CO2Et
OR OR
[H]–
H H
R= conditions yield dr
TBDPS 50 psi, Pt/Al2O3 96 96 : 4 H H
H '' 84 32 : 68 N N
TBDPS 92 65 : 35 H H
NaCNBH3, pH 4
H 90 67 : 33
''
CO2Et CO2Et
J. Am. Chem. Soc. 1983, 105, 1255.
22-hart-b 11/7/02 1:20 PM](https://image.slidesharecdn.com/gephyrotoxinevans-120416081537-phpapp02/75/Gephyrotoxin-21-2048.jpg)
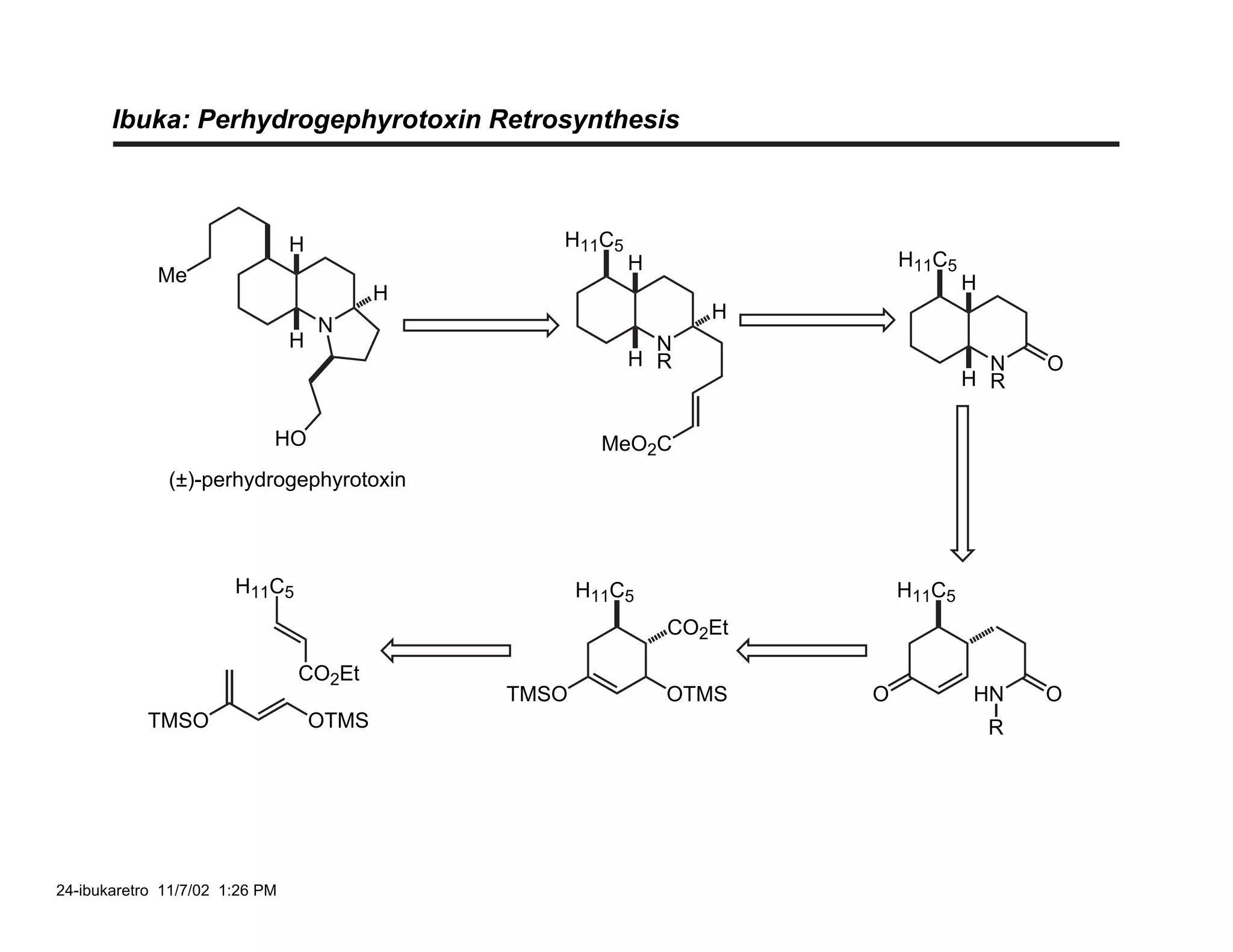
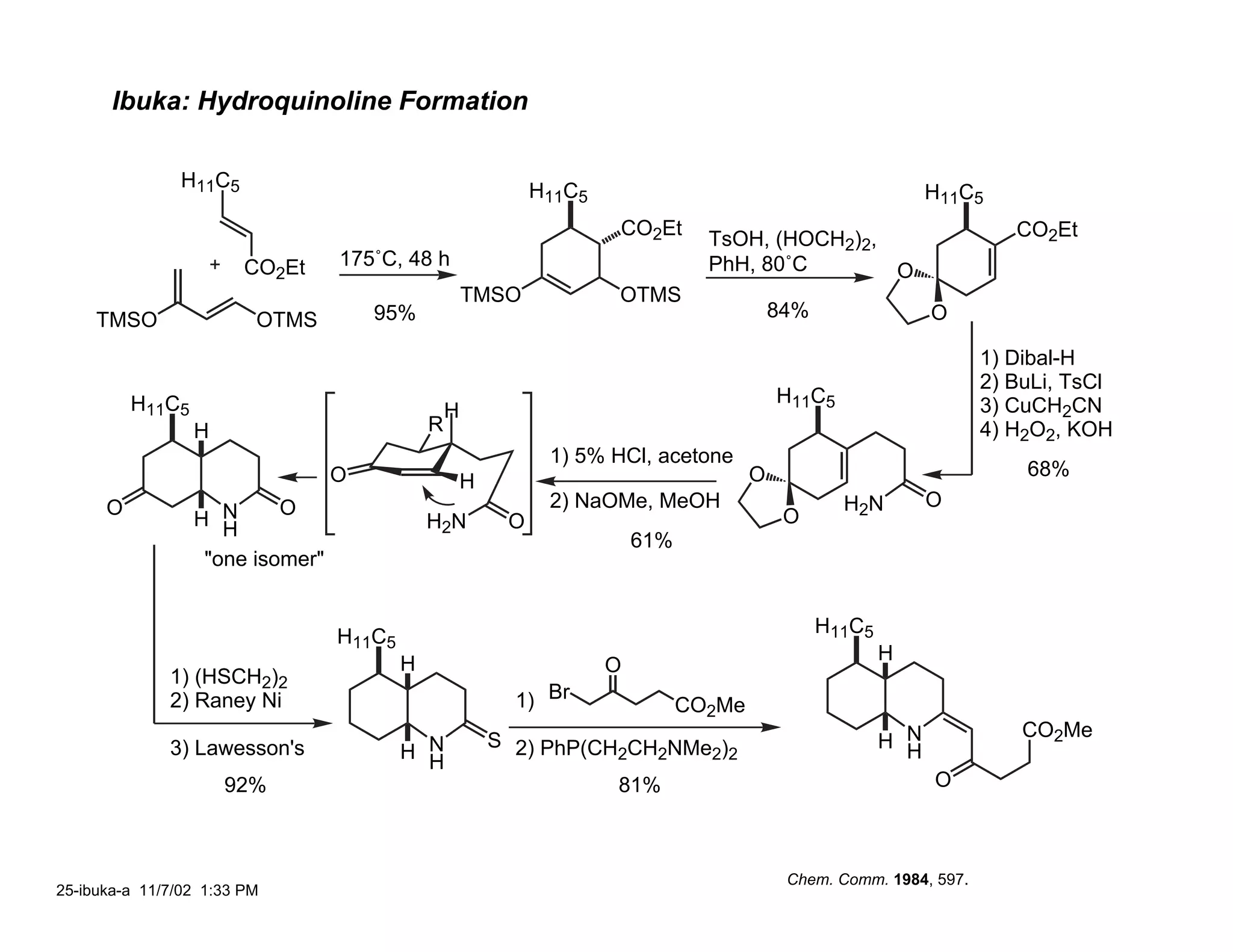
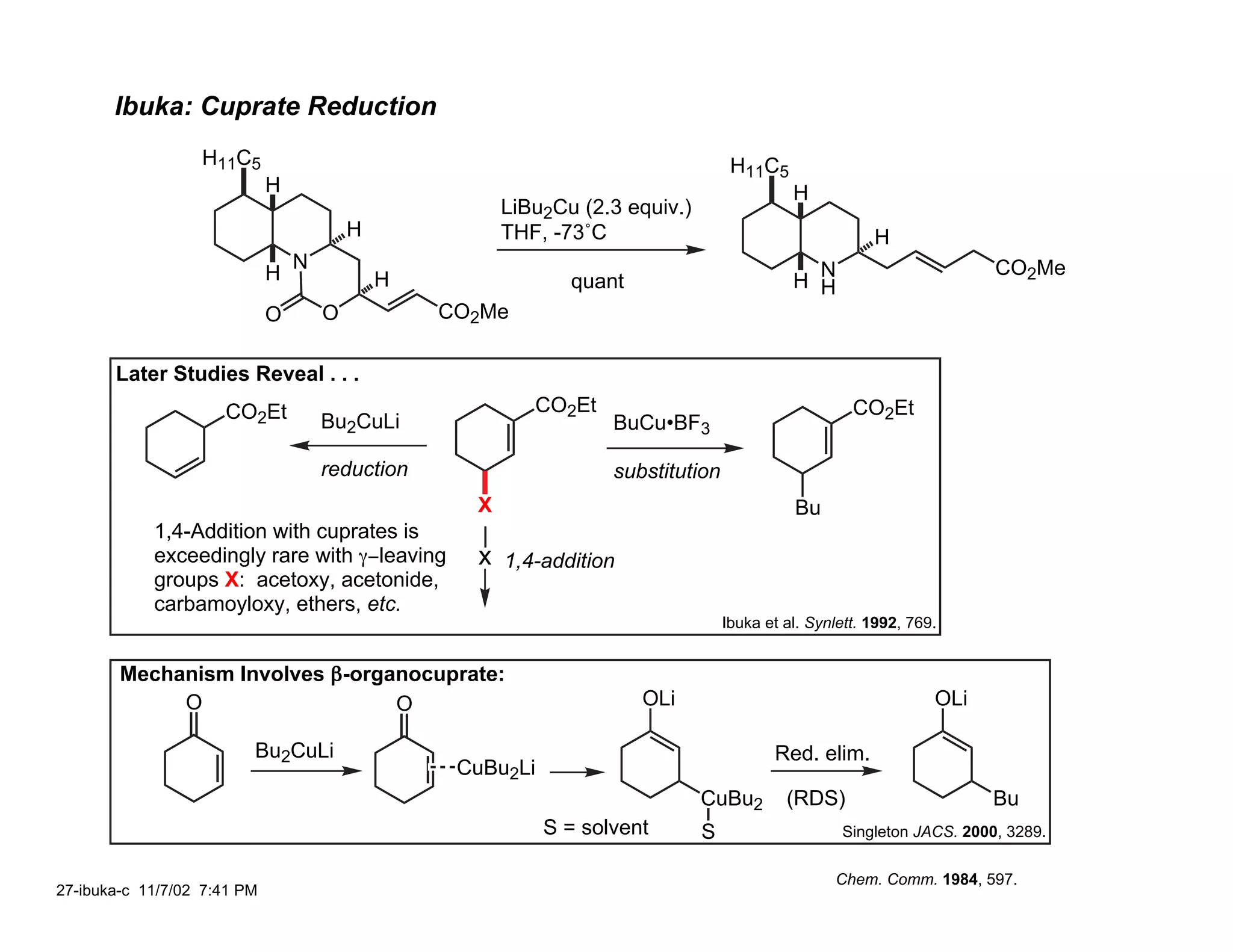
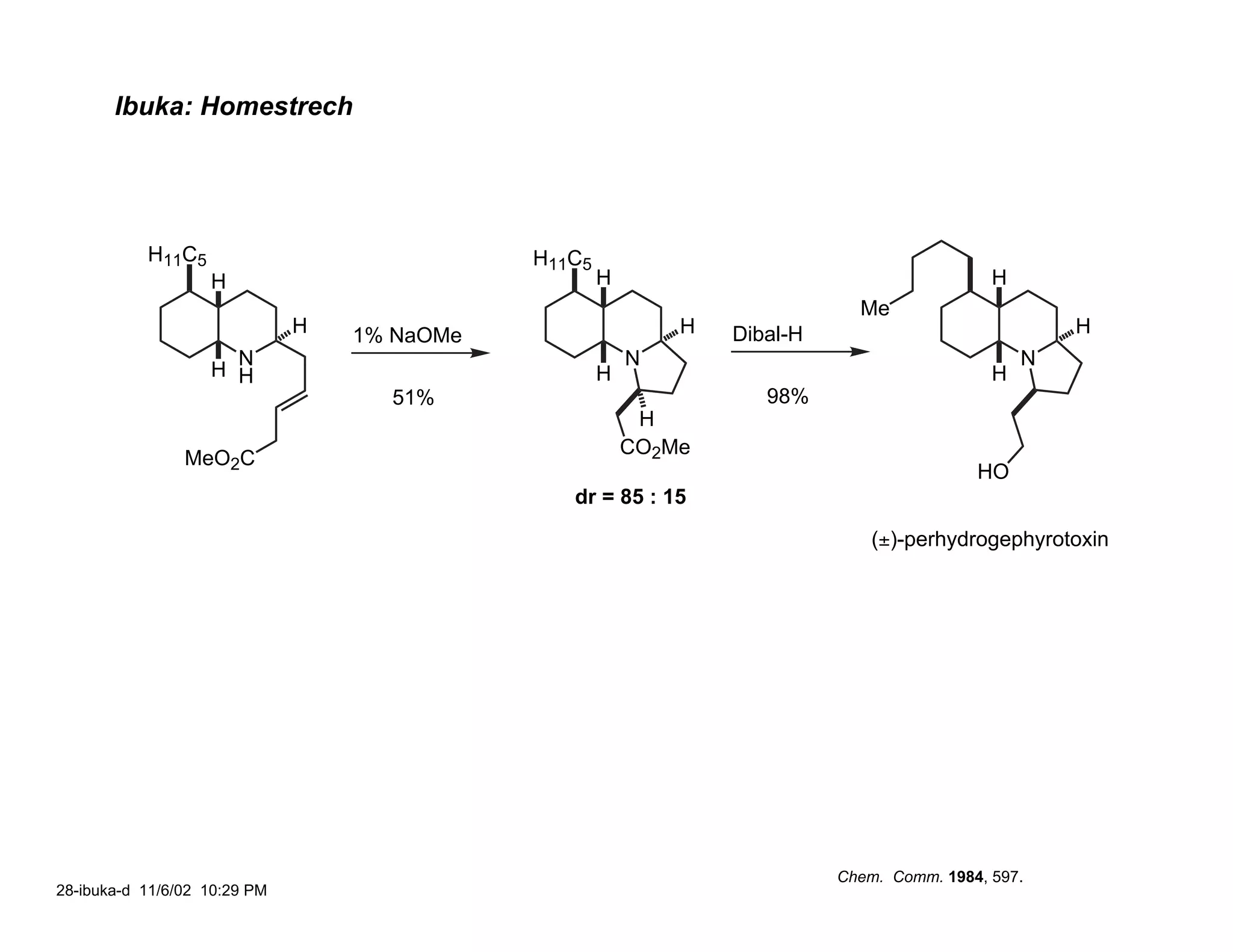
![Ibuka: C(3a) Stereochemistry
H11C5 H11C5 H11C5
H NaBH3CN H NEt3, MeOH H
pH 4, MeOH 65˚C
H H
H N
CO2Me 99% H N 3:1 H N
H
H H
O O O
MeO2C MeO2C
H11C5
H
H R
H L N
1) TsOH, PhH, 80˚C B O NaBH4
H N
H L
2) ClCO2Ph, pyr, DMAP CO2Me
HO H CO2Me [H]–
H11C5 Li H11C5
H 1) i-PrNChx
H
THF-HMPT; PhSeCl
H 2) LiOH H
99% H N H 3) CH2N2 H
N
H
PhO2C 4) H2O2, pyr
O O O CO2Me
65%
O
Chem. Comm. 1984, 597.
26-ibuka-b 11/7/02 9:37 PM](https://image.slidesharecdn.com/gephyrotoxinevans-120416081537-phpapp02/75/Gephyrotoxin-25-2048.jpg)
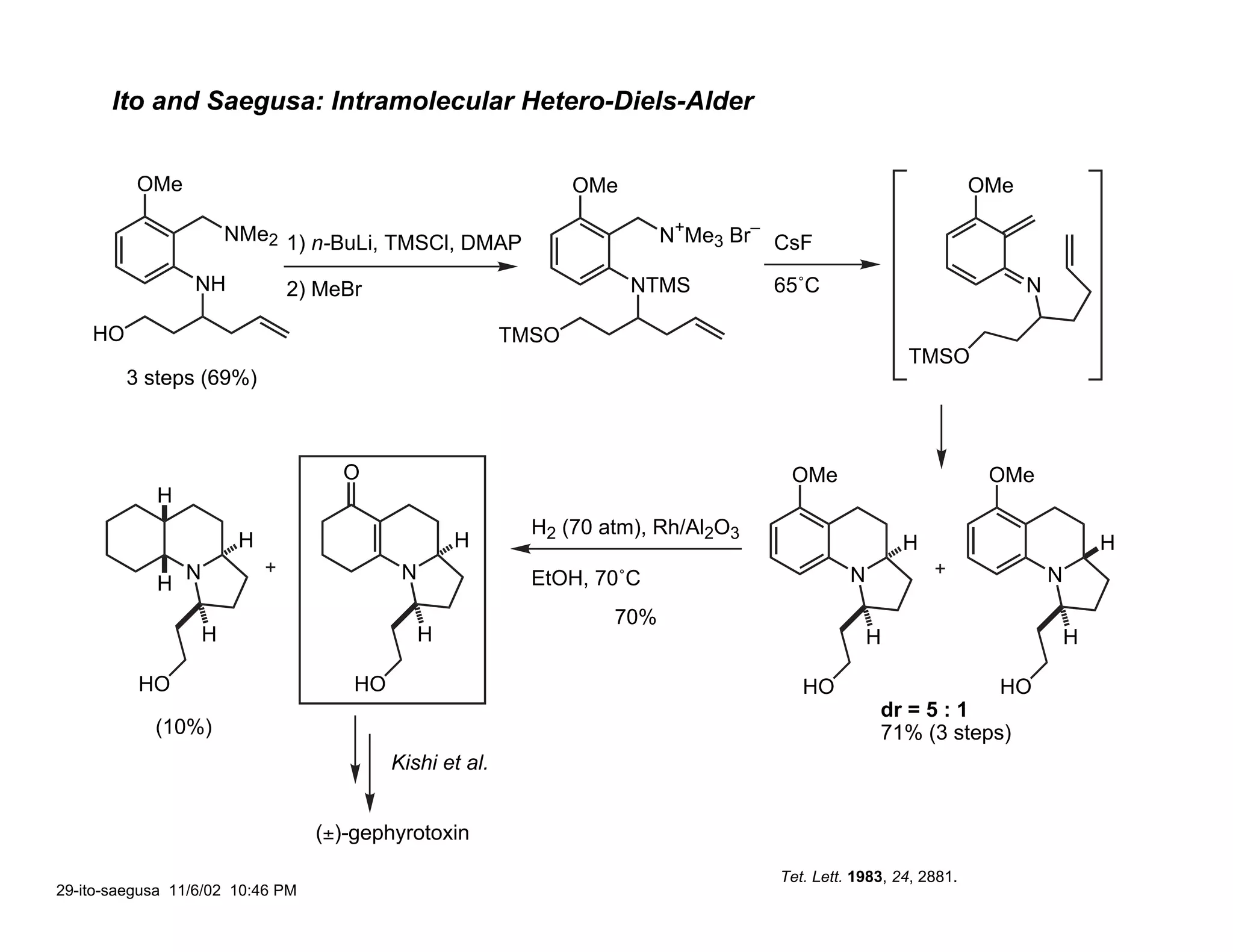
![Hsung: Intramolecular Formal [3+3]
O
O O O
OH
+
1) i. OH NH2–OAc
ii. MnO2
NH N
H2N
2) TBDPSCl, imid. PhMe 150˚ C 1-2 h
H
40% H
HO TBDPSO TBDPSO
48% (4 steps) via Noyori
asymmetric hydrogenation 1) H2, Pd/C
2) TBAF
O O
H
H H
H N N
H N Kishi et. al.
60 : 40
60%
HO HO
HO
(+)-gephyrotoxin extremely temperature sensitive:
high selectivity for undesired at 100˚ C
Hsung, R. Angew. Chem. Int. Ed. 2001, 40, 1516.
30-Hsung1 11/6/02 11:04 PM](https://image.slidesharecdn.com/gephyrotoxinevans-120416081537-phpapp02/75/Gephyrotoxin-29-2048.jpg)
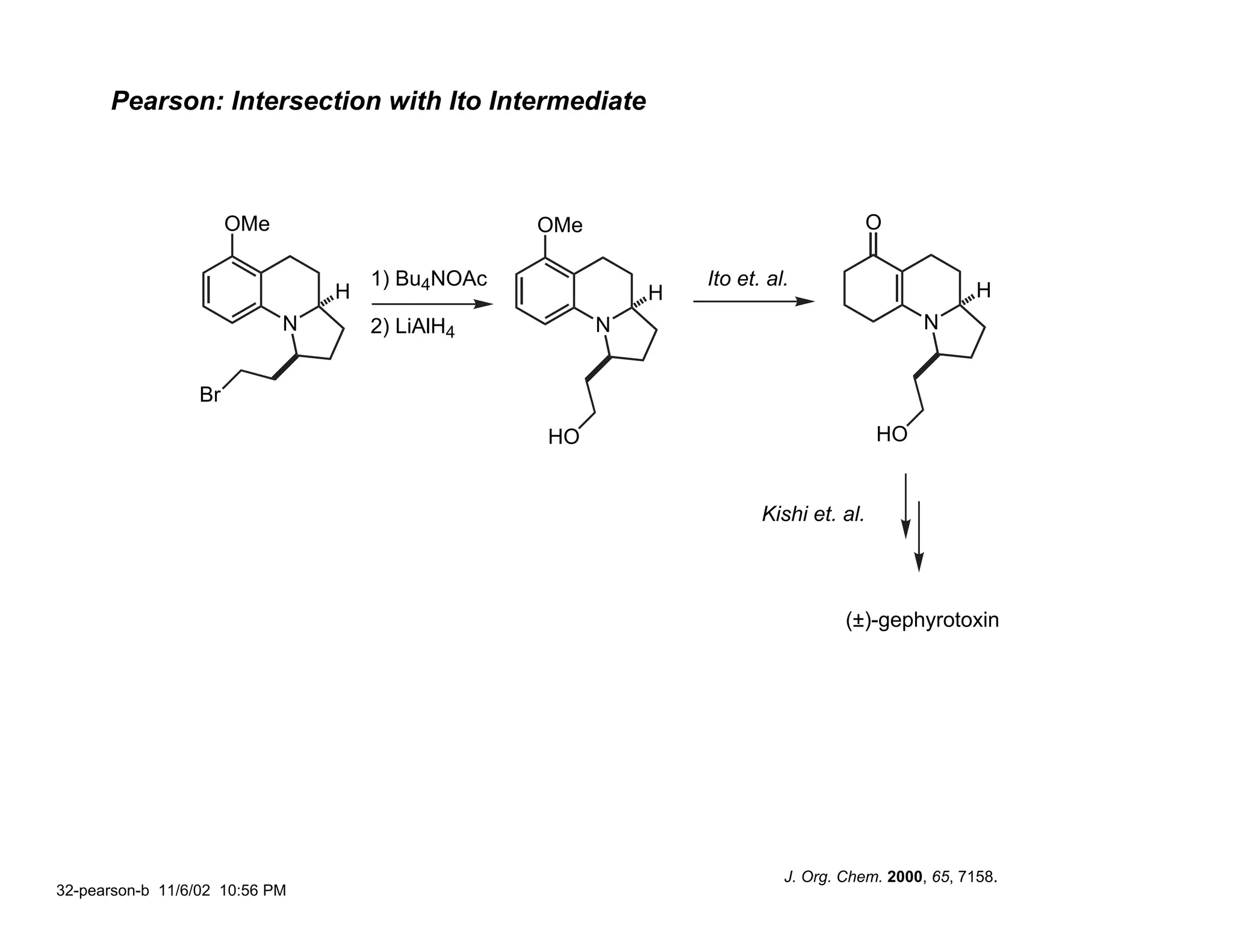
![Pearson: (±)-Gephyrotoxin via the Schmidt Reaction
OMe OMe OMe
5 - 7 steps TfOH
N3
N N N
O R
R
OMe OMe OMe OMe OMe
H [H–]
H
N N
N N N
H
R H R H R H R H R H
1 2 3
R / hydride reagent Yield 1 : 2: 3
CH2CH2Br / NaBH4 72% 39: 39: 22
/ Dibal-H 60% 52: 26: 22
/ L-selectride 55% 82: – : 18
CH2CH2OMOM – –
86% 16: 35: 49
CH2CH=CH2
J. Org. Chem. 2000, 65, 7158.
31-pearson-a 11/8/02 9:15 AM](https://image.slidesharecdn.com/gephyrotoxinevans-120416081537-phpapp02/75/Gephyrotoxin-30-2048.jpg)
![Synthesis of (±)-Gephyrotoxin: A Comparison
R2 R2 Steps: Total Yield (%):
H H
Overman (1983) [H]– 15 6.5
H
Reduction to concave face
N R1 N R1
O H O HH
H
H H
Kishi (1981) 24 2.8
N HN
Distal directed hydrogentation
HO H R HO H
H R
H
Hart (1983) H 22 1.8
N-acyliminium ion cyclization N N
H H
OMe O O
OMe
Ito (1983) N+Me3 Br- 7 + 12* = 19 7.4
Intramolecular [4+2]
H
NTMS N
R O O O
R
Hsung (2001) H 5 + 12* = 17 1.5
Formal [3+3] NH N
R OMe R
OMe
Pearson (2000) 8 + 12* = 20 3.5
Schmidt
N3 N
R
R H *from Kishi intermediate
33-geph-comp 11/4/02 4:03 PM](https://image.slidesharecdn.com/gephyrotoxinevans-120416081537-phpapp02/75/Gephyrotoxin-32-2048.jpg)