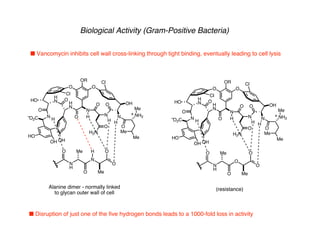
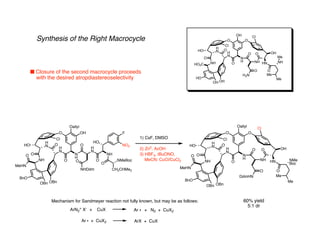
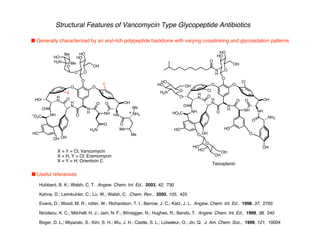
The document discusses the structural features and biosynthesis of vancomycin-type glycopeptide antibiotics, detailing their complex synthesis and the challenges involved. It highlights the biological activity of vancomycin against gram-positive bacteria and outlines various synthetic methodologies, including chiral auxiliary techniques and macrocyclizations. Key references and strategies for the successful completion of the vancomycin structure are also provided.

![Proposed Biosynthesis of Vancomycin-Type Glycopeptides
! Remarkably, genes and proteins responsible for the biosynthesis of these molecules have been characterized
! Biosynthesis can be reduced to peptide elongation and post-translational modification
Cl OH OH Cl
MeO OMe O Me
OH HO OH OH
HO H2N HO Me
NHMe OH OH
H2N OH OH OH H2N OH H2N
H2N H2N H2N O H2N
O O
O O O O
OH
OH
Cl OR
Cl OR Cl
HO OH
O O
H Cl
HO N O H
H O O OH HO N O
N [O] H O O OH
O N Me N
O N Me
H NH HN NH2
O2C NH O H NH HN NH2
O2C NH O
O O
O O
H2N Me
HO H2N Me
Me HO
OH OH Me
OH OH
! The challenge to the synthetic chemist is immense: biosynthesis entails 35 total steps](https://image.slidesharecdn.com/vancomycin-120416015702-phpapp02/85/Comparative-syntheses-of-Vancomycin-3-320.jpg)