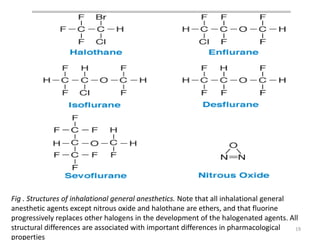
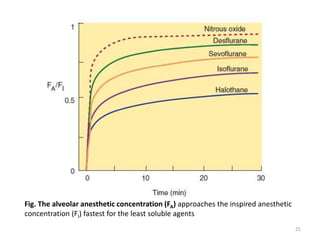
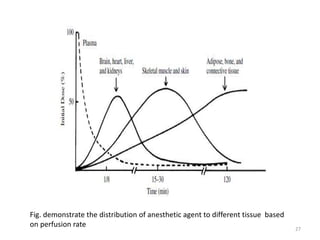
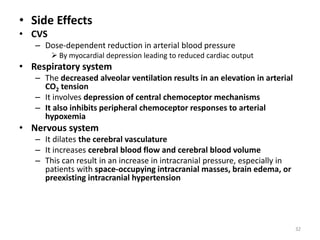
This document discusses drugs that act on the central nervous system (CNS). It covers general classifications of CNS drugs including general anesthetics, local anesthetics, opioids, and others. It focuses on general anesthetics, describing their mechanisms, stages of anesthesia, effects on cardiovascular and respiratory systems, and ideal properties. General anesthetics are divided into intravenous and inhalational agents. Inhalational agents include gases like nitrous oxide and volatile liquids that are administered via inhalation to induce anesthesia.