Embed presentation
Downloaded 24 times





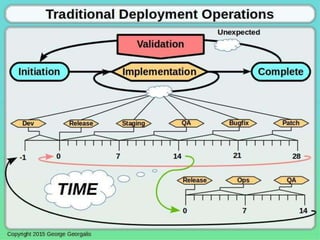

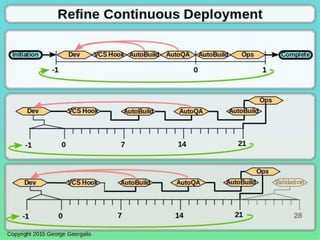

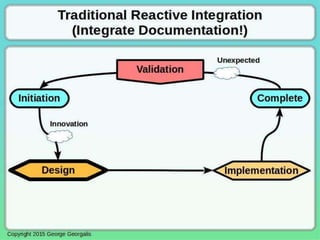
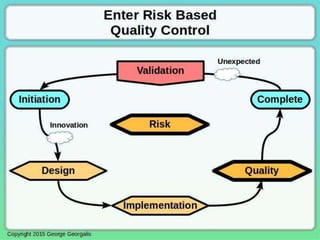
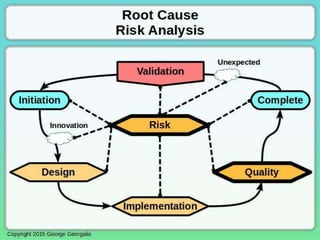
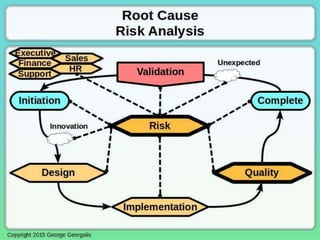
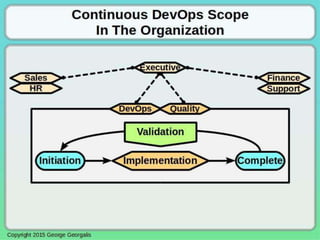
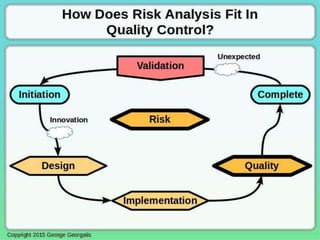
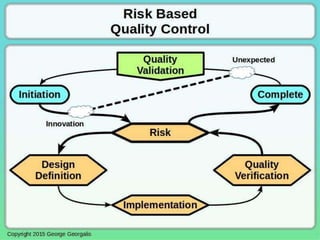
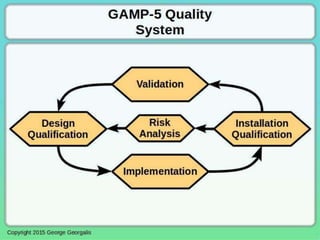
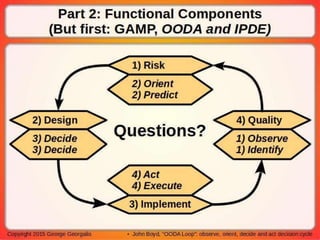
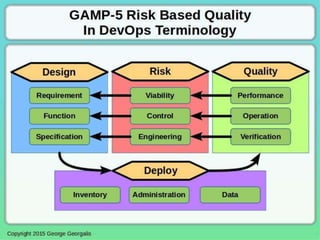


This document discusses applying principles from GAMP-5, which provides guidance for quality assurance in pharmaceutical manufacturing, to development operations using agile and continuous deployment methods. It proposes taking a risk-based approach to ensure compliance and readiness when using agile and continuous deployment practices for software development and operations.



















