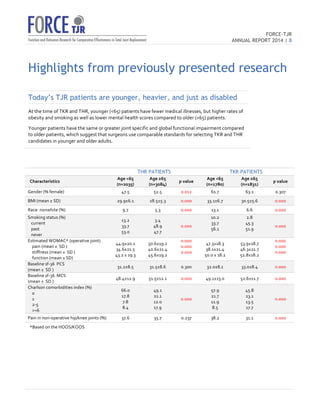
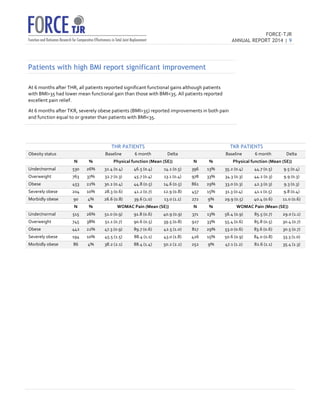
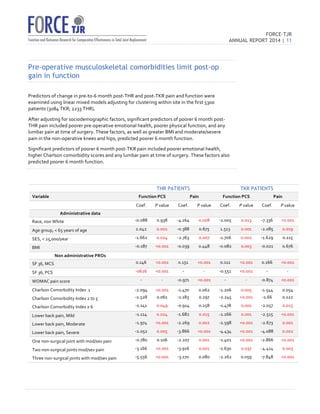
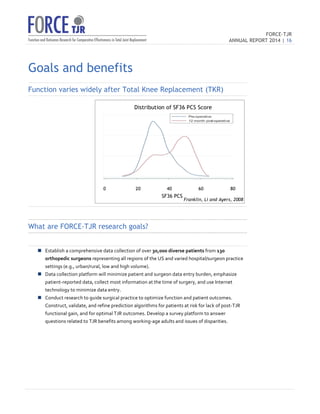
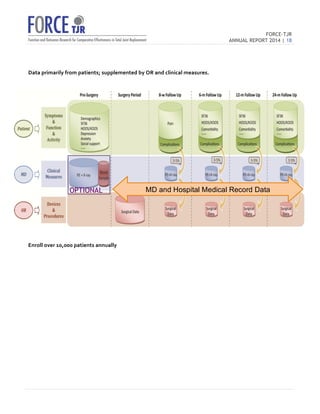
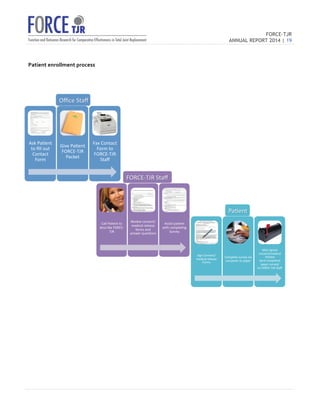
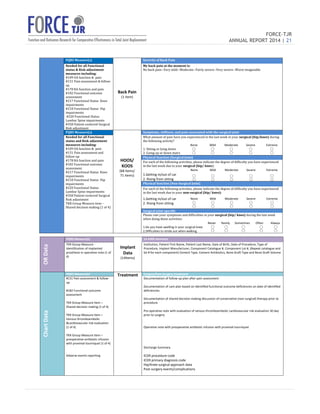
The 2014 FORCE-TJR annual report outlines the establishment and progress of a national total joint replacement registry initiated by the University of Massachusetts Medical School. As of June 2014, over 20,000 patients have been enrolled to collect comprehensive data on patient-reported outcomes and complications, supporting quality improvement and research in orthopedic care. The registry not only assists surgeons in comparing practice outcomes but also facilitates collaborations for better patient selection and care in line with regulatory standards.




![FORCE-TJR
ANNUAL REPORT 2014 | 5
TJR
o Patient
self-‐reported
Pre-‐operative
25th,
50th,
and
75th
percentile
pain
and
function
scores
are
remarkably
consistent
across
surgeons
in
FORCE
suggesting
comparable
indications
for
surgery.
o While
greater
BMI
is
a
risk
factor
for
peri-‐operative
complications,
FORCE-‐
TJR
found
that
at
6
months
after
total
hip
or
knee
replacement,
patients
with
a
BMI
higher
than
35,
also,
reported
significant
gains
in
pain
relief
and
physical
function.
o The
burden
of
musculoskeletal
comorbidities-‐
specifically
moderate
or
severe
pain
in
the
lumbar
spine
and
non-‐operative
hips
and
knees-‐
negatively
affects
self-‐reported
function
at
6
months
after
surgery.
Future
public
comparisons
of
PROs
after
TJR
must
be
cautious
to
adjust
for
co-‐existing
musculoskeletal
conditions.
Patricia
D.
Franklin,
MD
MBA
MPH
David
C.
Ayers,
MD
PI
FORCE-‐TJR
Chair,
National
Stakeholder
Committee
Map of Participating Core Centers and Community Sites
WY
CO
WA
OR
Core Clinical Centers
UMass Medical School, Worcester, MA
Connecticut Joint Replacement Institute, Hartford, CT
The University of Rochester Medical Center, Rochester, NY
Medical University of South Carolina, Charleston SC
Baylor College of Medicine, Houston, TX
PA
VA
VT NH ME
Community Sites currently enrolled
ID
MT ND
MN MI
MI
SD
NE
KS
TX LA
AL GA
SC
NC
NY
MA
CT RI
NJ
DE
MD
DC
WV
FL
MS
OK
IA
MO
IL
IN
OH
KY
TN
WI
AR
NV UT
AZ NM
CA
Community Sites
It’s important to
participate [in FORCE-TJR]
so that people who
have knee replacements in
the future can benefit from
my experience.
Patient participant,
Michael L., age 53
(knee replacement) MA
“
”](https://image.slidesharecdn.com/force-tjrannualreport2014-141124133301-conversion-gate01/85/Force-TJR-Annual-Report-2014-5-320.jpg)




![FORCE-TJR
ANNUAL REPORT 2014 | 14
TJR
Quarterly
MD
Report
This
executive
summary
of
the
quarterly
surgeon
report
addresses
3
questions:
1. How
do
my
patients
compare
to
patients
at
other
sites
on
key
risk-‐adjustment
factors?
[Patient
Mix]
2. How
do
my
patients
compare
to
other
sites
on
pre-‐TJR
pain
and
function?
[Patient
Selection
and
Timing
of
Surgery]
3. How
do
my
risk-‐adjusted
6
and
12
month
pain
and
function
compare
to
other
sites?
[TJR
patient-‐reported
outcomes]](https://image.slidesharecdn.com/force-tjrannualreport2014-141124133301-conversion-gate01/85/Force-TJR-Annual-Report-2014-14-320.jpg)



![FORCE-TJR
ANNUAL REPORT 2014 | 23
TJR
Appendix 1: FORCE-TJR Bibliography (through
June 2014)
PUBLICATIONS
1. Franklin
PD,
Lewallen
D,
Bozic
K,
Hallstrom
B,
Jiranek
W,
Ayers
D.
Implementation
of
patient-‐reported
outcomes
in
US
total
joint
replacement
registries:
rationale,
status,
and
plans.
The
Journal
of
Bone
Joint
Surgery.
ICOR
suppl
(in
press)
2. Gandek
B.
Measurement
properties
of
the
Western
Ontario
and
McMaster
Universities
Osteoarthritis
Index:
A
systematic
review”.
Arthritis
Care
Research.
(Hoboken).
2014
Jul
21.
doi:
10.1002/acr.22415.
[Epub
ahead
of
print]
3. Ayers
DC,
Li
W,
Harrold
LR,
Allison
JA,
Franklin
PD.
Pre-‐operative
pain
and
function
profiles
reflect
consistent
TKR
patient
selection
among
US
surgeons.
Clinical
Orthopaedics
and
Related
Research.
Clinical
Orthopaedics
and
Related
Research.
2014;
Jun
2014
Epub
ahead
of
print
DOI
10.1007/s11999-‐014-‐3716-‐5
4. Ayers
DC
and
Franklin
PD.
Hip
Outcome
Assessment.
In
Callaghan
JJ,
Rosenberg
AG,
Rubash
HE,
editors.
The
Adult
Hip
(Callaghan,
Aaron,
Rubash)
Lippincott
Williams
Wilkins;
2014.
5. Devers
K,
Gray
B,
Ramos
C,
Shah
A,
Blavin
F,
Waidmann
T.
Key
Informant
Interview:
Patricia
Franklin,
MD,
University
of
Massachusetts
Medical
School
(FORCE-‐TJR).
In
ASPE
Report:
The
Feasibility
of
Using
Electronic
Health
Data
for
Research
on
Small
Populations;
2013.
6. FORCE-‐TJR
In:
An
Introduction
to
AHRQ's
Third
Edition
of
Registries
for
Evaluating
Patient
Outcomes.
AHRQ
2013.
7. Franklin
PD,
Harrold
LR,
Ayers
DC.
Incorporating
patient
reported
outcomes
in
total
joint
arthroplasty
registries:
challenges
and
opportunities.
Clinical
Orthopaedics
and
Related
Research.
2013;
471(11):3482-‐
3488.
PMCID:
PMC3792256
8. Ayers
DC.
Zheng
H,
Franklin
PD.
Integrating
Patient-‐Reported
Outcomes
(PROs)
into
orthopedic
clinical
practice:
proof
of
concept
from
FORCE-‐TJR.
Clinical
Orthopaedics
and
Related
Research.
2013;
471(11):3419-‐
3425.
PMCID:
PMC3792269
9. Franklin
PD,
Rosal
MC.
Can
knee
arthroplasty
play
a
role
in
weight
management
in
knee
osteoarthritis?
Arthritis
Care
Research
2013
May;
65
(5):
667–668.
10. Franklin
PD,
Allison
JJ,
Ayers
DC.
Beyond
implant
registries:
a
patient-‐centered
research
consortium
for
comparative
effectiveness
in
total
joint
replacement.
JAMA.
2012
Sep;
308(12):
1217-‐8.
PRESENTATIONS
AT
INTERNATIONAL
AND
NATIONAL
MEETINGS
1. Franklin
PD,
Harrold
L,
Li
W,
Ash
A,
Ayers
DC.
Improving
risk
prediction
models
for
readmission:
adding
clinical
variables
to
administrative
data.
International
Congress
of
Arthroplasty
Registries,
Boston,
MA.
(June
2014)
2. Ayers
DC,
Harrold
L,
Li
W,
Noble
P,
Allison
JJ,
Franklin
PD.
Pre-‐op
THR
and
TKR
pain
and
functional
limitation
profiles
are
consistent
across
U.S.
surgeons.
International
Congress
of
Arthroplasty
Registries,
Boston,
MA.
(June
2014)
(Podium)
3. Franklin
PD,
Harrold
L,
Li
W,
Allison
JJ,
Lewis
C,
Ayers
DC.
Are
all
important
predictors
of
pain
and
function
after
TKR
and
THR
included
in
registry
data?
International
Congress
of
Arthroplasty
Registries,
Boston,
MA.
(June
2014)](https://image.slidesharecdn.com/force-tjrannualreport2014-141124133301-conversion-gate01/85/Force-TJR-Annual-Report-2014-23-320.jpg)




