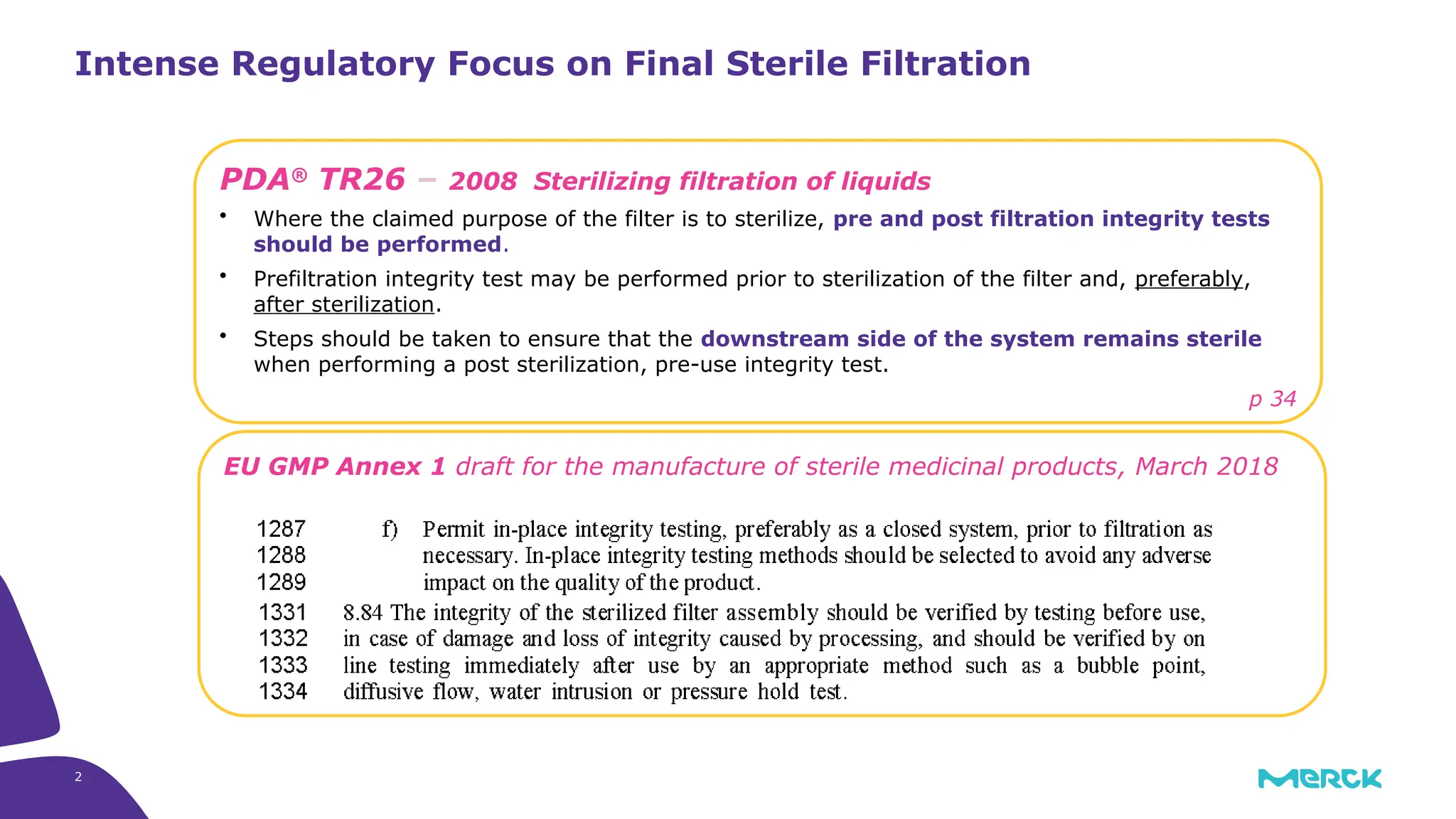
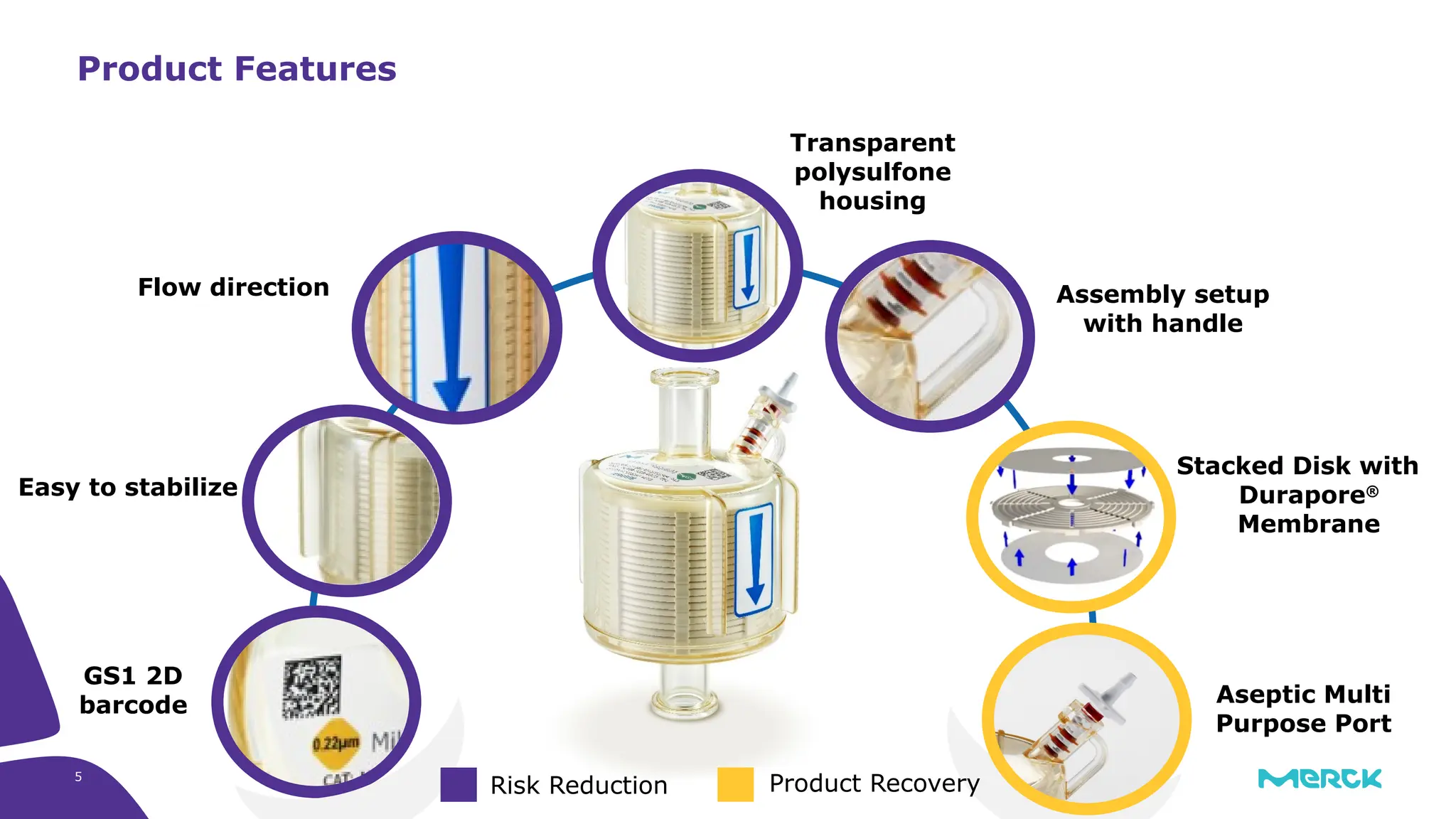
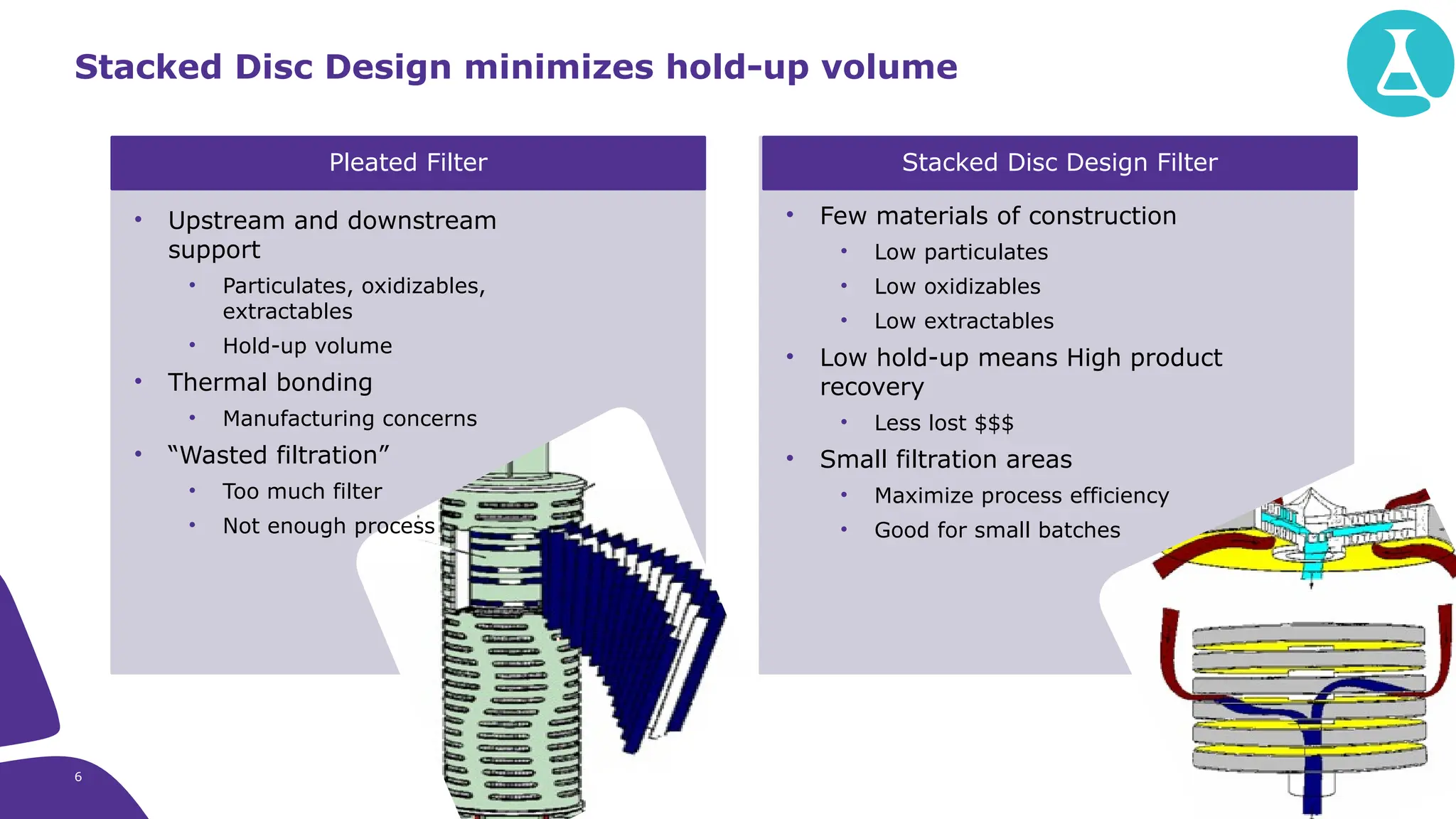
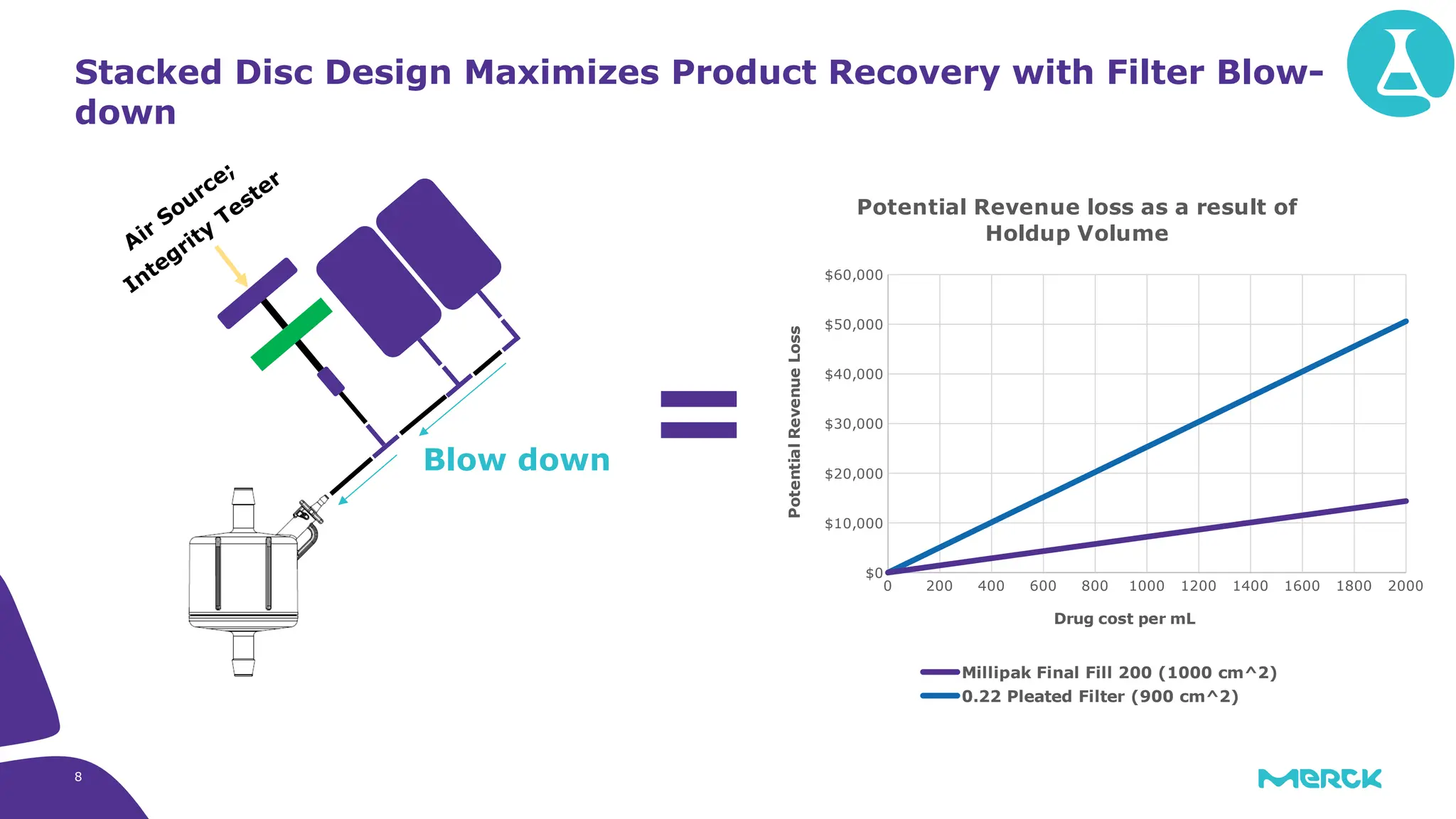
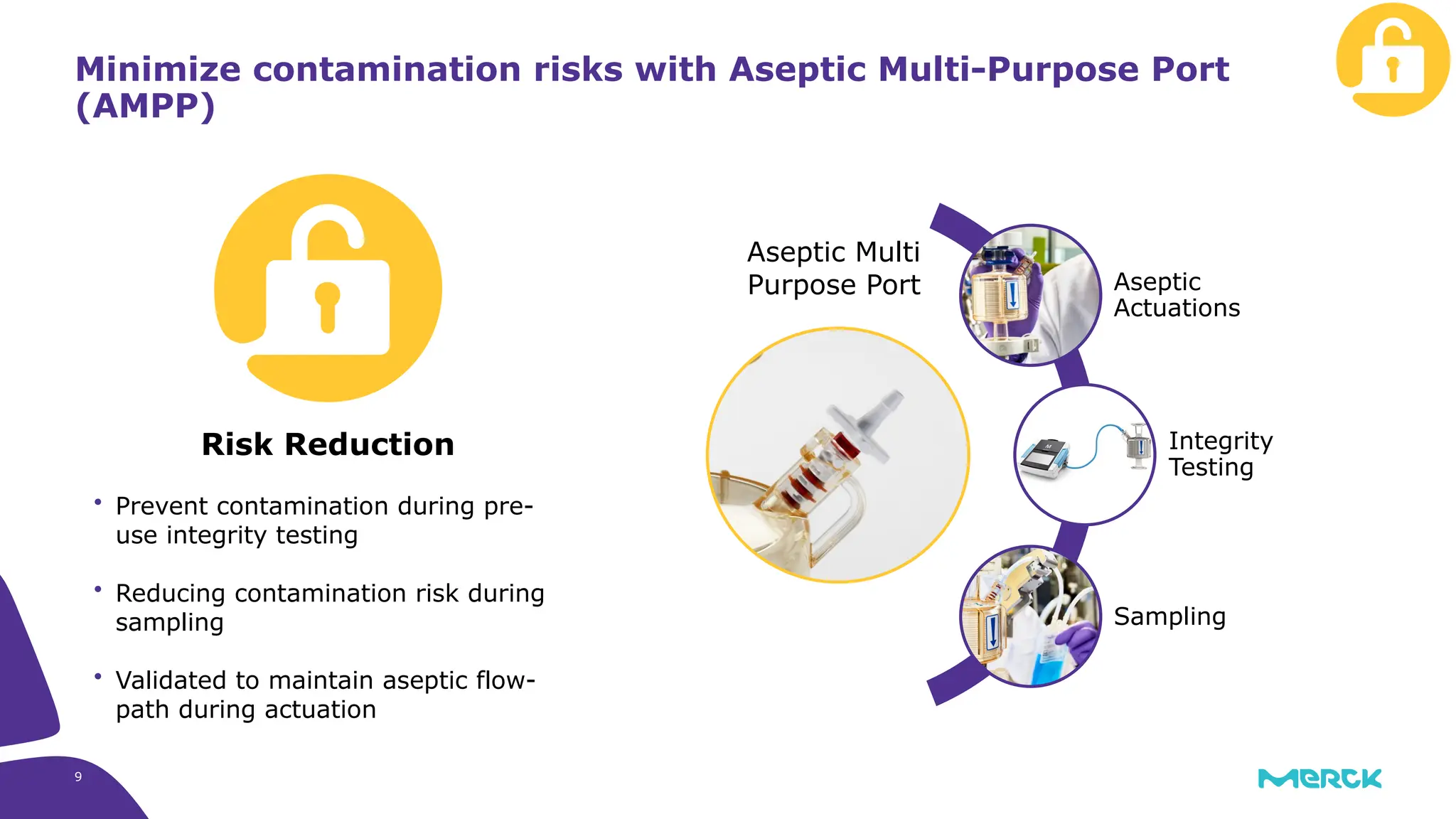
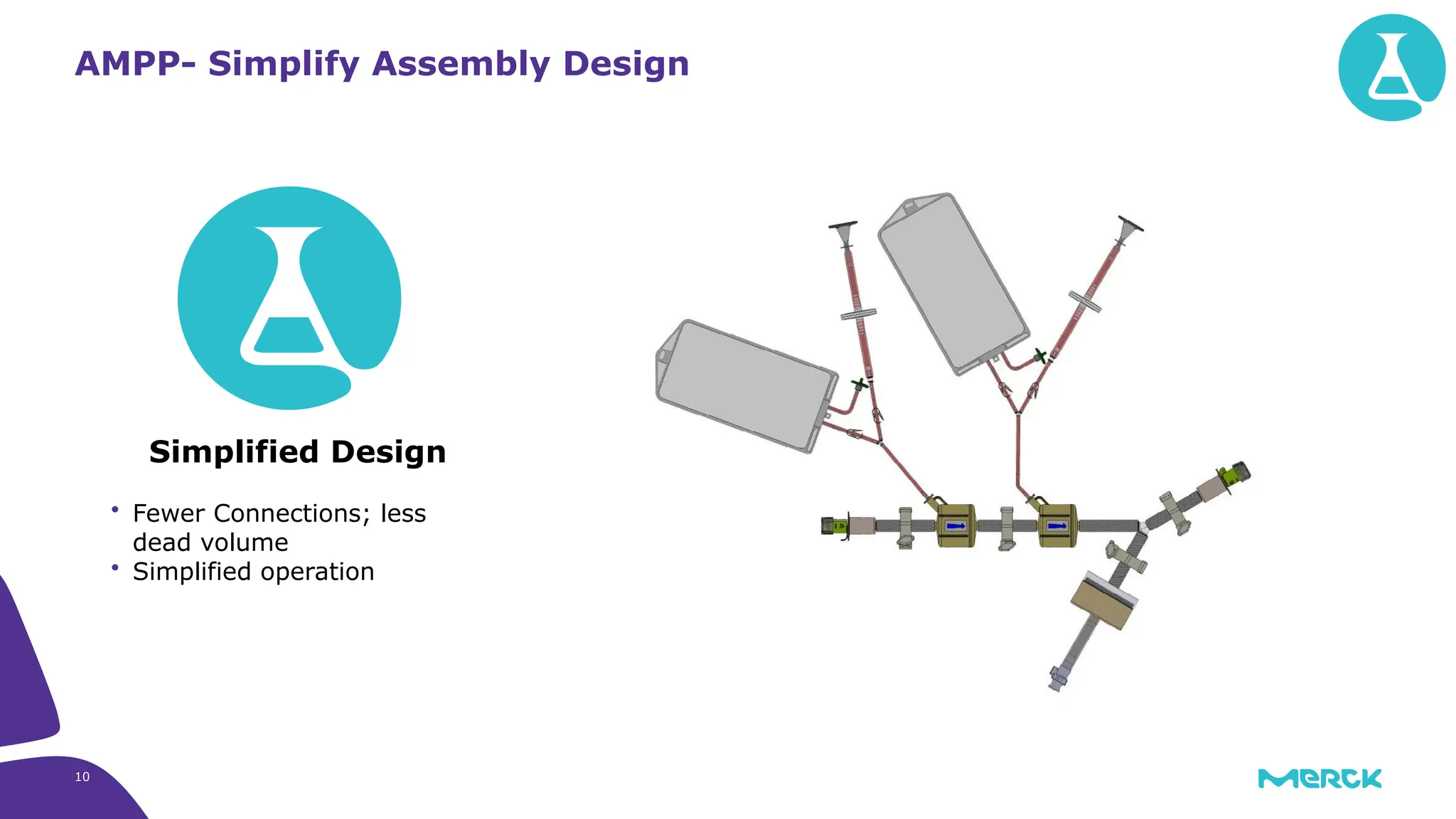
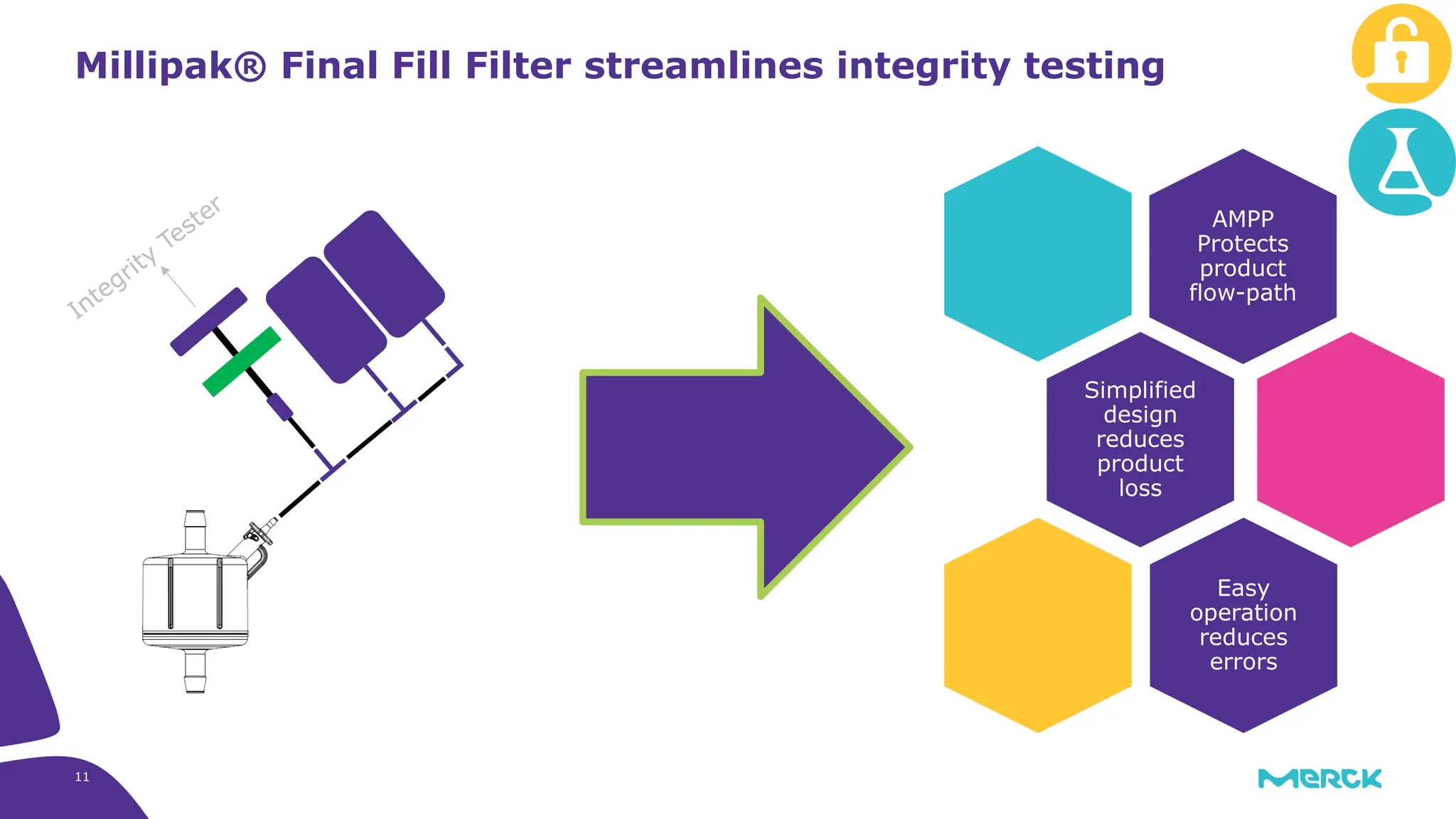
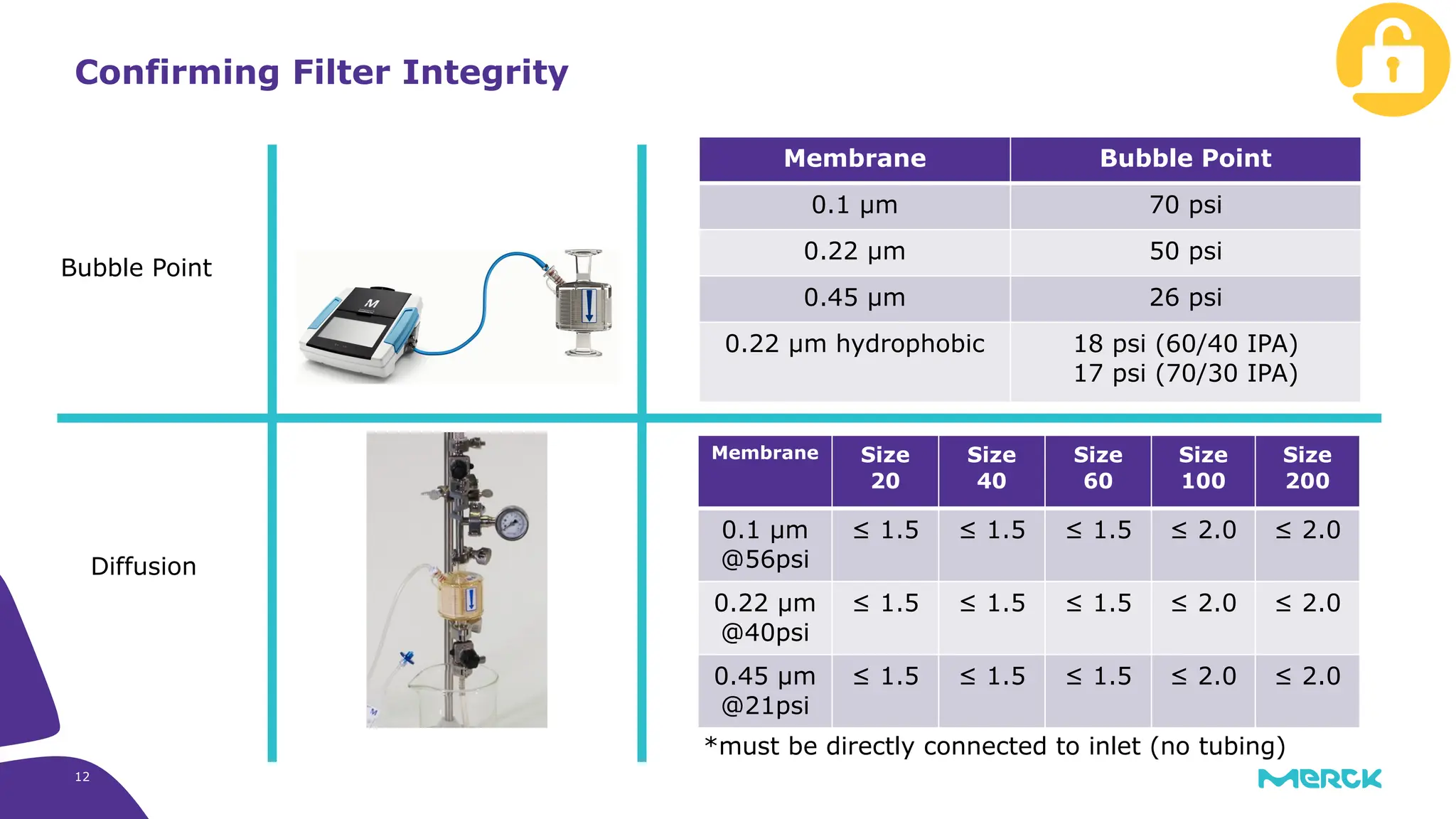
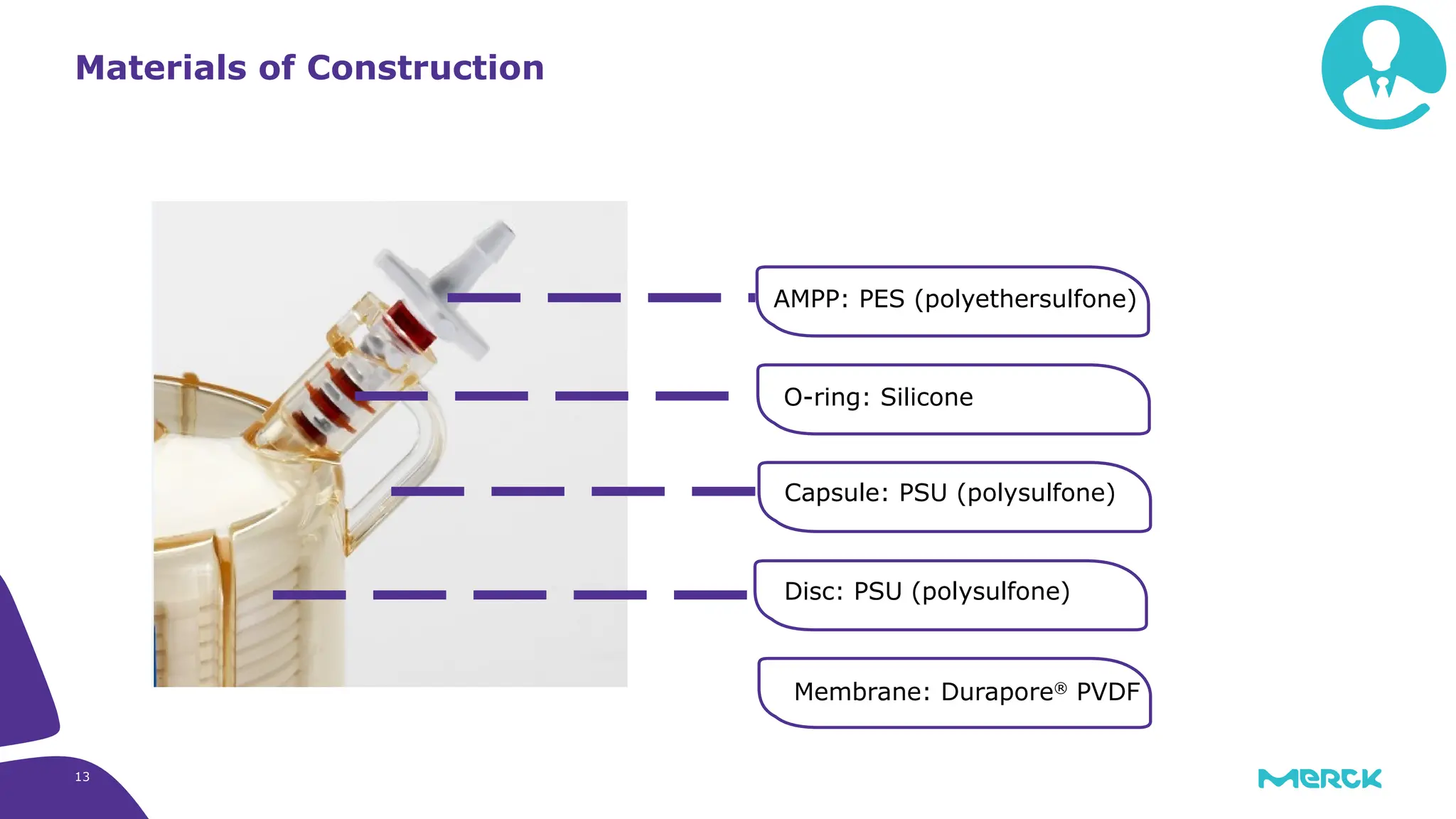
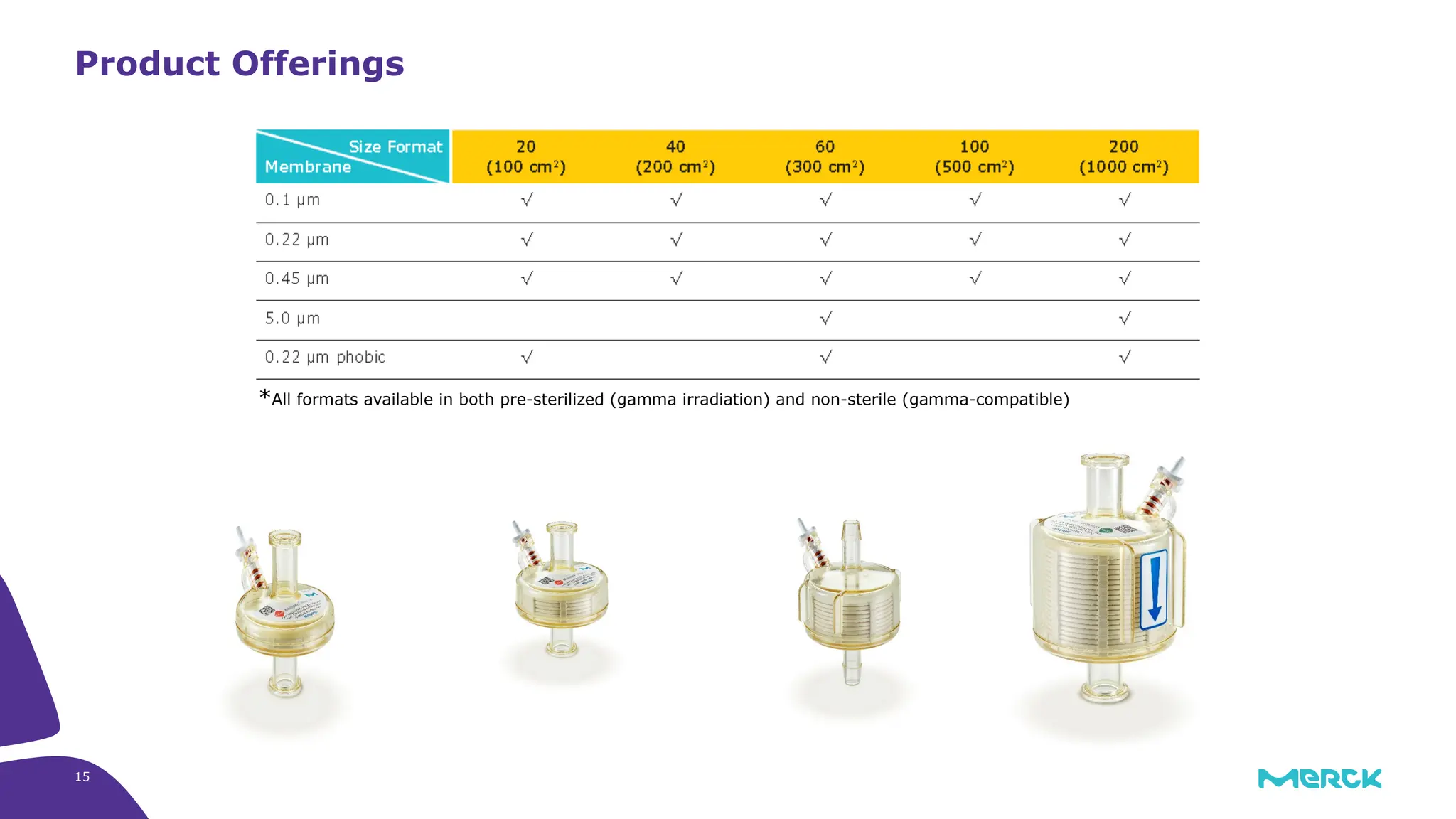
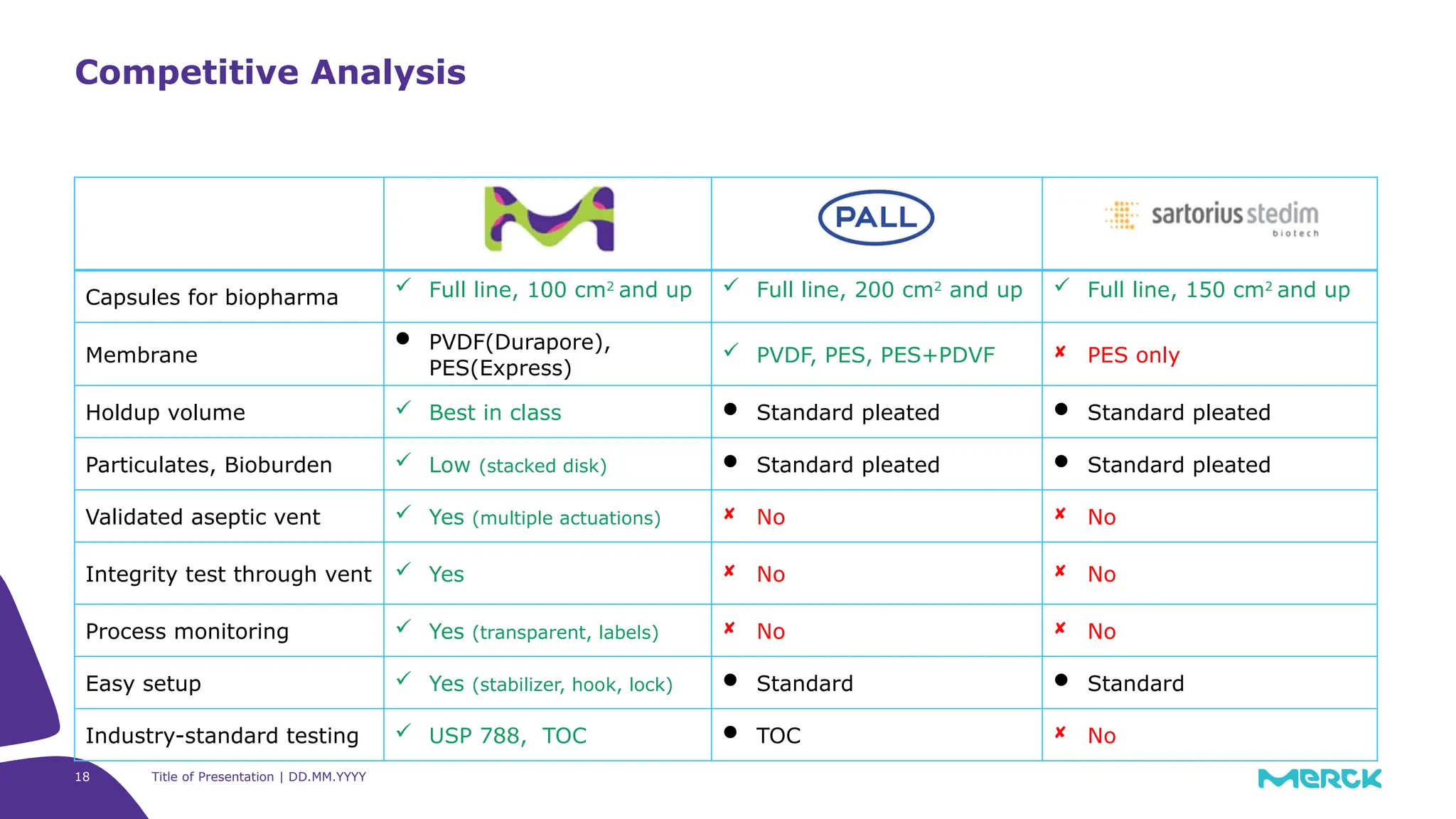
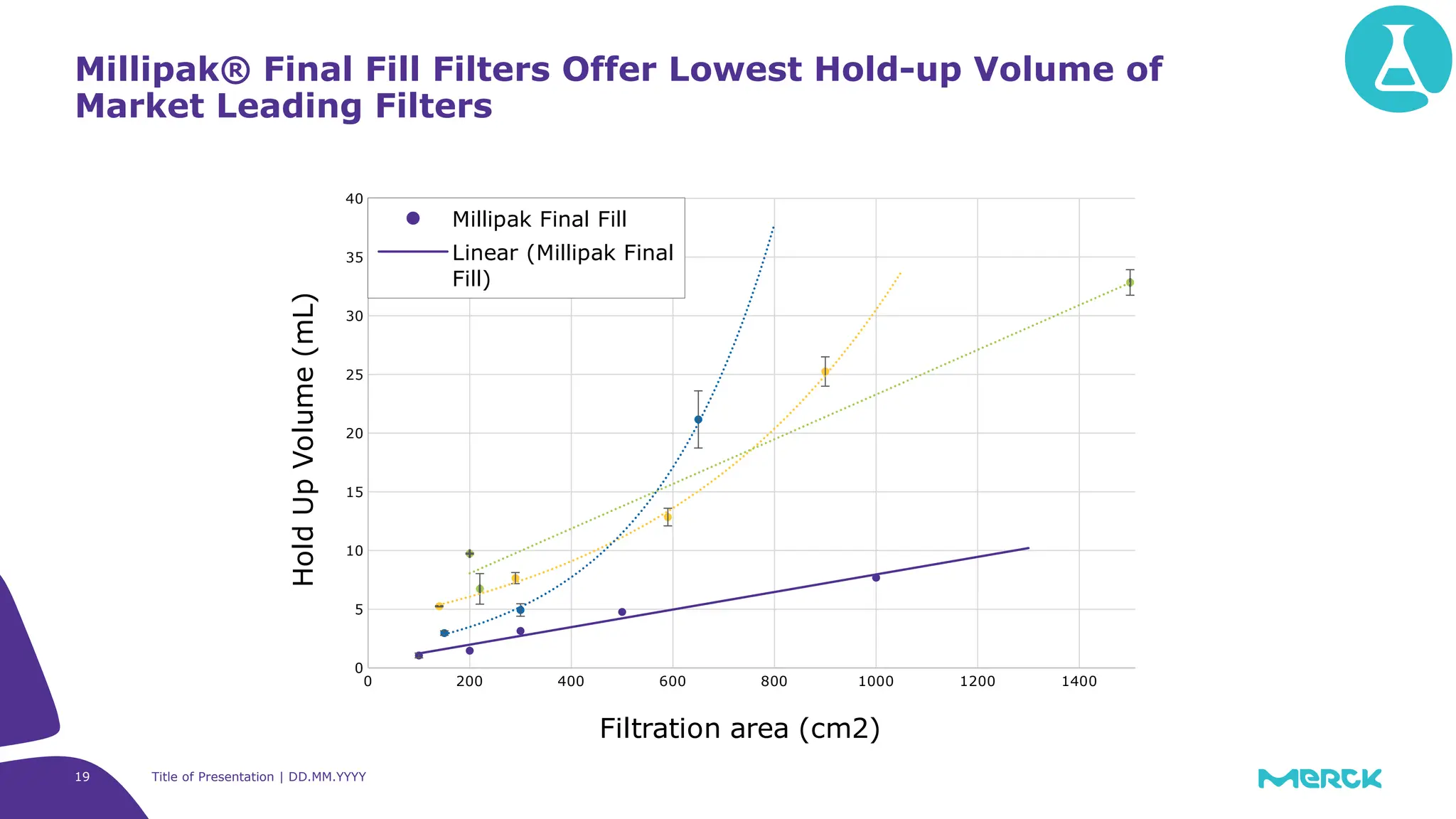
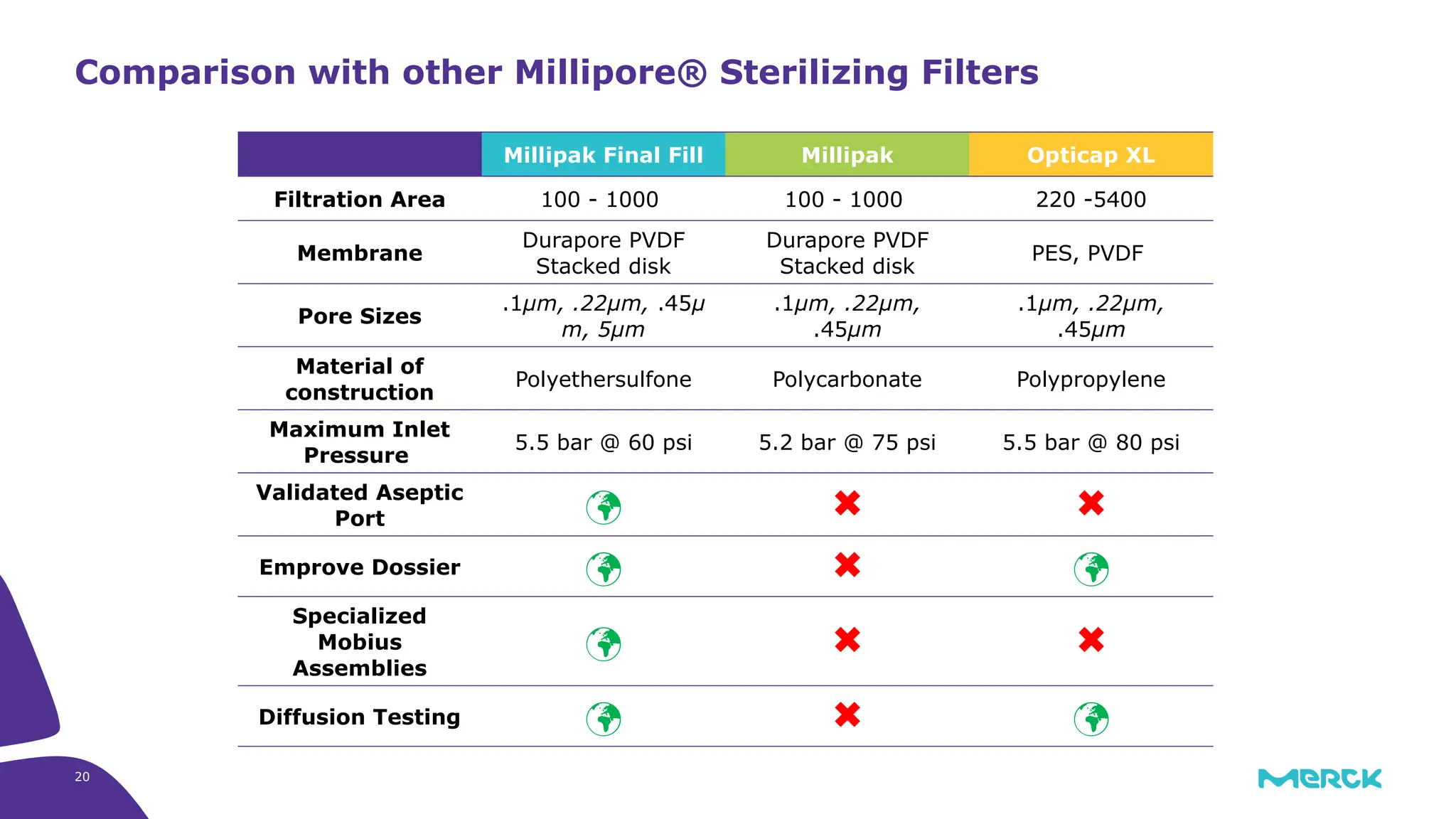
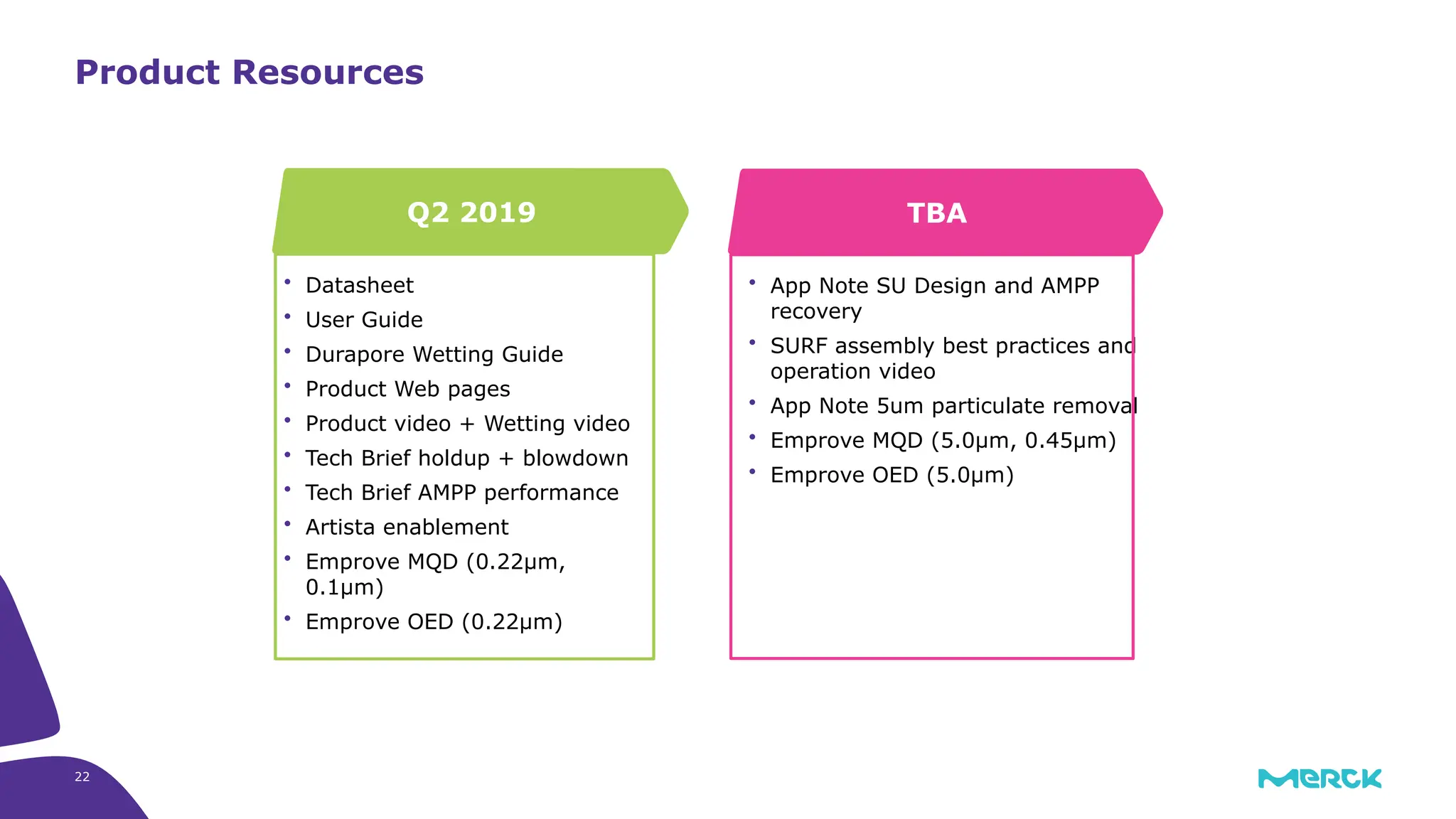
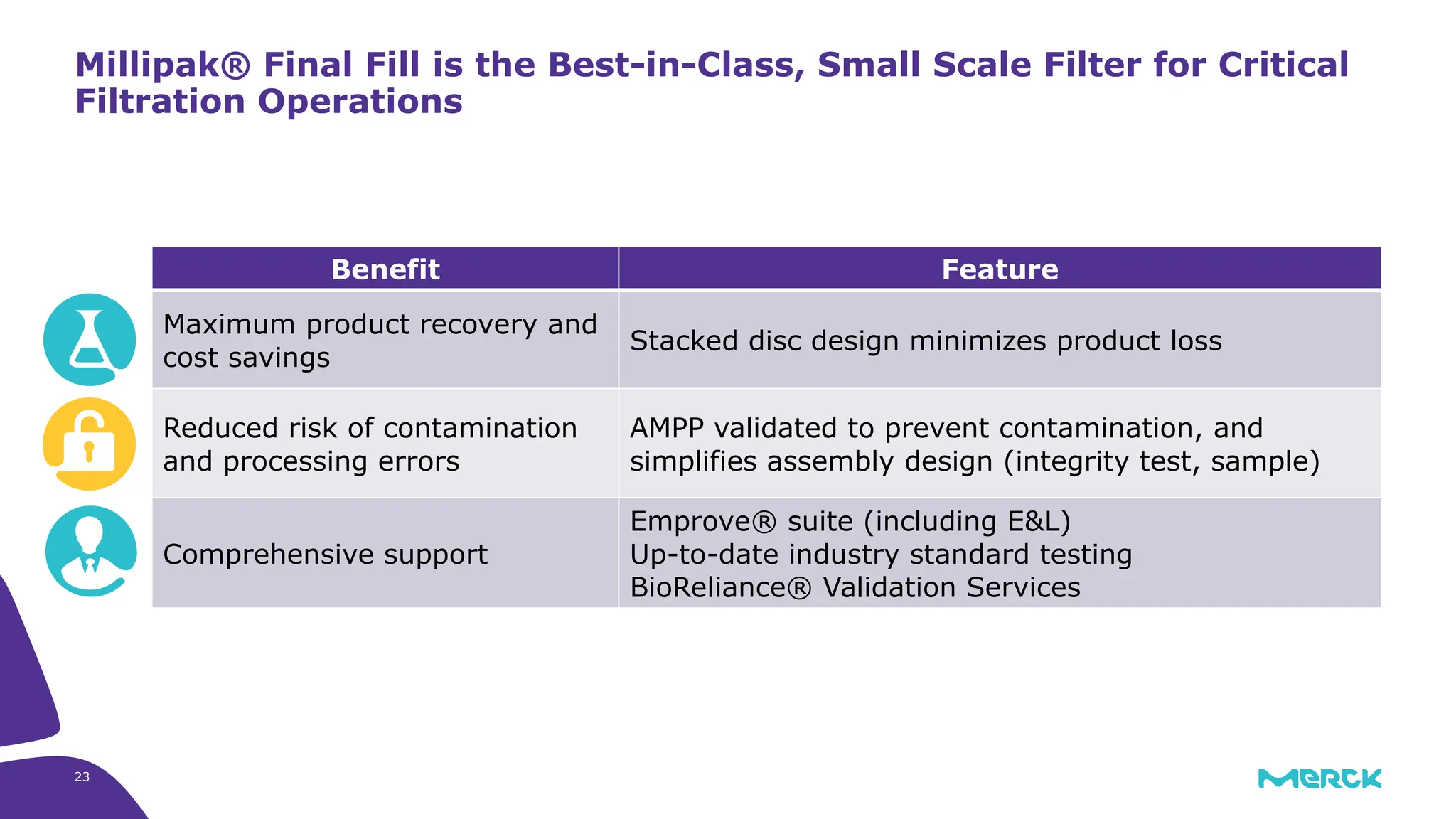
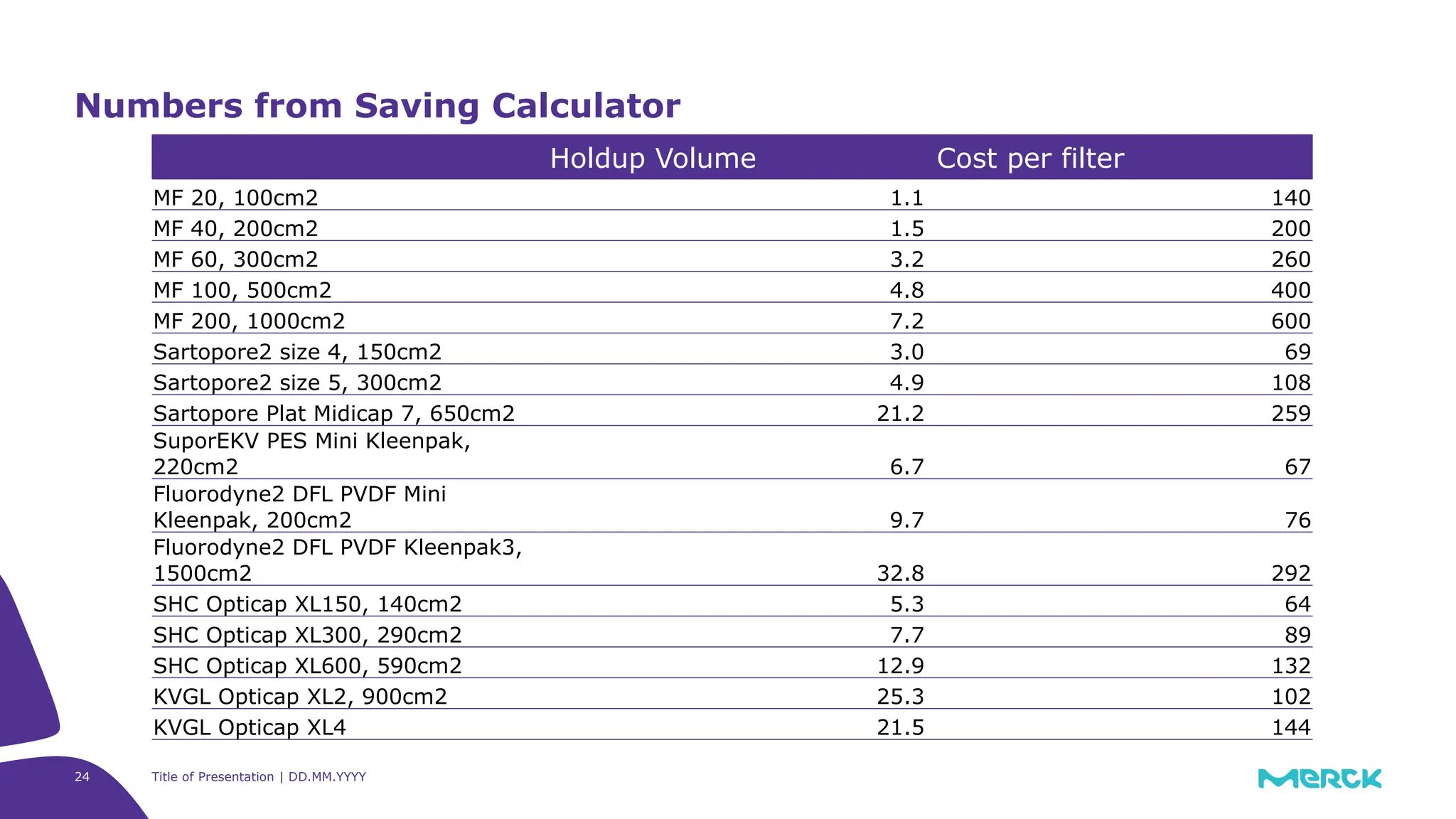
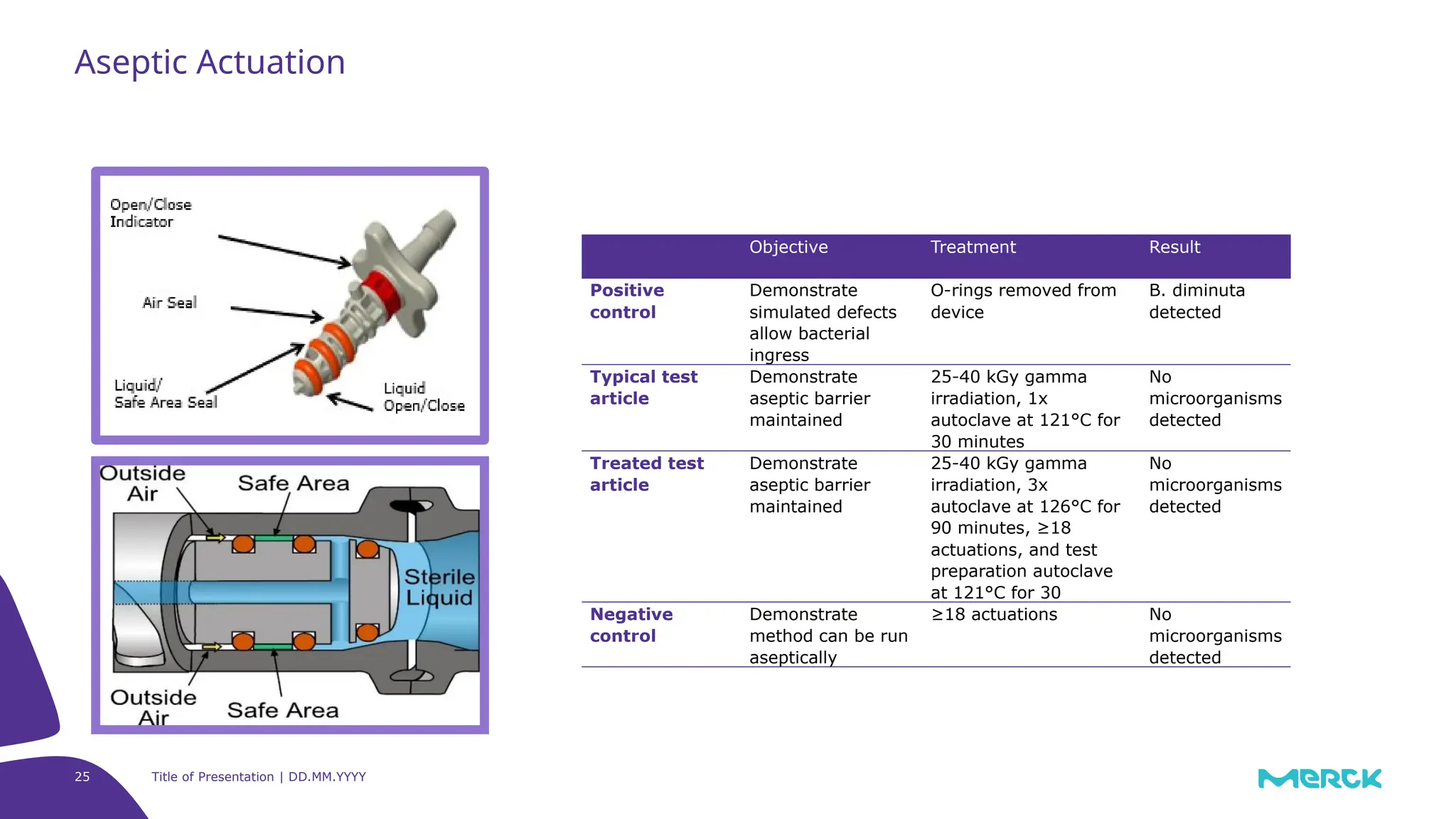
The millipak® final fill filter is designed to maximize product recovery and enhance protection in sterile filtration of high-value products, meeting stringent regulatory standards. Its stacked disc design minimizes hold-up volume, reduces contamination risk with an aseptic multi-purpose port, and offers comprehensive support through the emprove® suite. This filter is characterized by a proven durapore® membrane that ensures low protein binding and high flow rates, making it ideal for critical filtration operations.