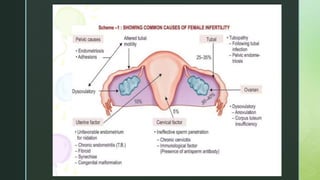
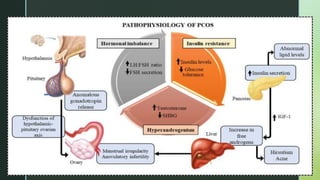
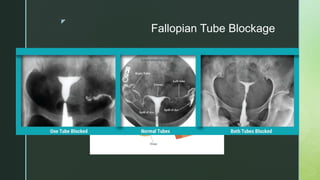
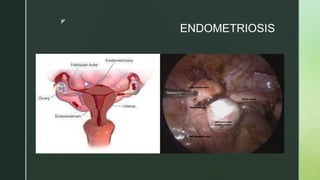
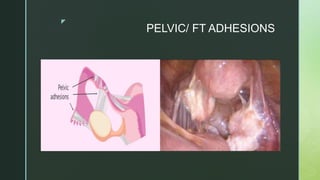
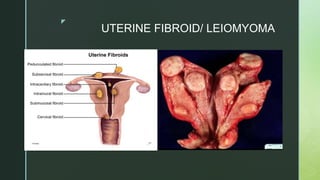
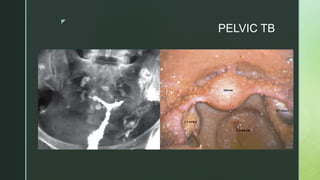
This document discusses the causes of female infertility. It notes that the most common identifiable factors of female infertility are ovulatory disorders (25%), endometriosis (15%), pelvic adhesions (12%), tubal blockage (11%), and other tubal/uterine abnormalities (11%). It describes various pathophysiological factors that can cause female infertility including congenital abnormalities, hormonal issues like anovulation, endometriosis, pelvic adhesions, tubal blockage, and uterine anomalies.










![z
Hypergonadotropic Hypoestrogenic
Anovulation
Premature ovarian failure (POF) is the cessation of ovarian function before 40 years of age. The
term refers to the condition when the ovaries have lost their germinative and hormonal functions
because of the exhaustion of the number of ovarian follicles prior to the typical age for
physiological menopause,
The medical history of patients with POF usually reveals a normal age of menarche and regular
menstrual cycles, followed by oligomenorrhoea or sudden amenorrhoea. In some cases,
secondary loss of menses is diagnosed after stopping contraceptive pills . Most frequently,
women suffer from hot flushes, excessive sweating, hair loss, as well as skin and mucous
membrane dryness.
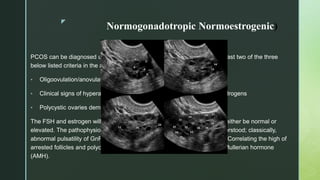
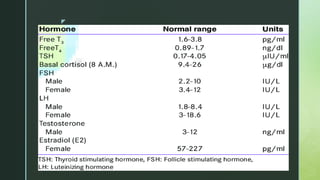
Tests reveal a hypergonadotropic-hypogonadic hormone profile which is characterised by low
oestradiol (E2) levels (< 20 pg/ml), elevated gonadotropin levels (follicle-stimulating hormone
[FSH] > 20 IU/l), low anti-Müllerian hormone (AMH) levels – < 0.5 ng/ml (< 1 ng/ml), and low
inhibin B levels](https://image.slidesharecdn.com/ppt-230103094810-755e22cb/85/Female-infertility-and-its-causes-19-320.jpg)












![z
REFERENCES
Walker MH, Tobler KJ. Female Infertility. [Updated 2022 May 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from:
https://www.ncbi.nlm.nih.gov/books/NBK556033/
Williams Gynecology, Third Edition John O. Schorge, Lisa M. Halvorson, Joseph I. Schaffer · 2016 ·
EPIDEMIOLOGY : Chandra A, Copen CE, Stephen EH. Infertility and impaired fecundity in the United States, 1982-2010: data from the National Survey of Family Growth. Natl Health Stat Report. 2013
Aug 14;(67):1-18, 1 p following 19. [PubMed]
Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS
Med. 2012;9(12):e1001356. [PMC free article] [PubMed]
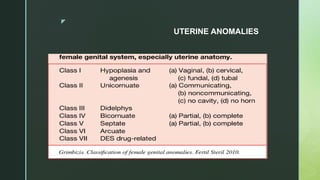
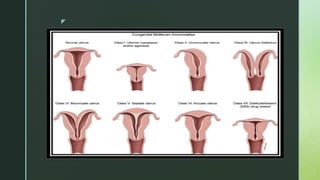
Grimbizis, Grigoris F. and Rudi Campo. “Congenital malformations of the female genital tract: the need for a new classification system.” Fertility and sterility 94 2 (2010): 401-7 .
Jankowska K. Premature ovarian failure. Prz Menopauzalny. 2017 Jun;16(2):51-56. doi: 10.5114/pm.2017.68592. Epub 2017 Jun 30. PMID: 28721130; PMCID: PMC5509972.
Infertility Workup for the Women's Health Specialist: ACOG Committee Opinion, Number 781. Obstet Gynecol. 2019 Jun;133(6):e377-e384. [PubMed]
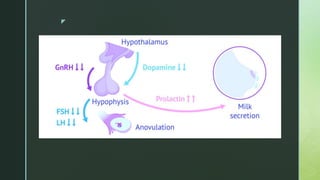
Seppälä M, Ranta T, Hirvonen E. Hyperprolactinaemia and luteal insufficiency. Lancet. 1976 Jan 31;1(7953):229-30. [PubMed]
Comprehensive Gynecology Rogerio A. Lobo, David M Gershenson, Gretchen M Lentz · 2016
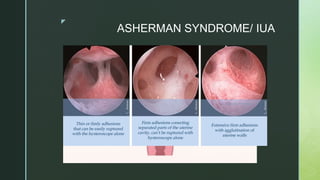
Charles M. March, INTRAUTERINE ADHESIONS, Obstetrics and Gynecology Clinics of North America, Volume 22, Issue 3,1995,
K Dreyer, R van Eekelen, RI Tjon-Kon-Fat, JW van der Steeg, P Steures, MJC Eijkemans, F van der Veen, PGA Hompes, BWJ Mol, N van Geloven,The therapeutic effect of
hysterosalpingography in couples with unexplained subfertility: a post-hoc analysis of a prospective multi-centre cohort study, Reproductive BioMedicine Online,Volume 38, Issue
2,2019,
Jeshica Bulsara, Priyanshi Patel, Arun Soni, Sanjeev Acharya,A review: Brief insight into Polycystic Ovarian syndrome,Endocrine and Metabolic Science,Volume 3,2021,](https://image.slidesharecdn.com/ppt-230103094810-755e22cb/85/Female-infertility-and-its-causes-39-320.jpg)