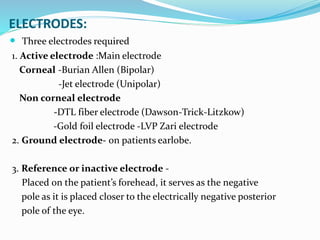
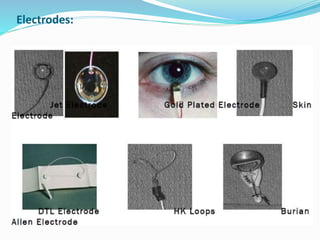
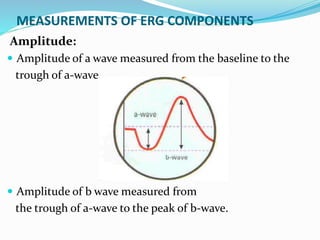
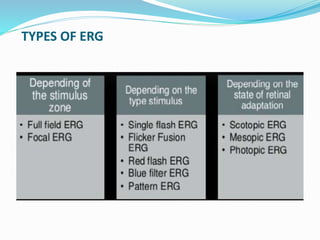
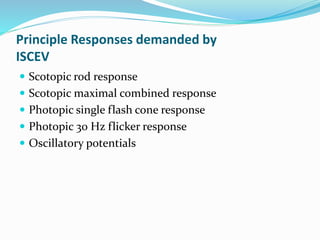
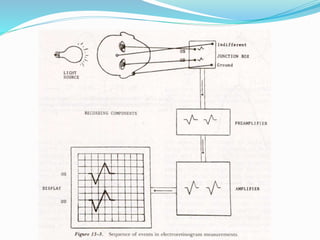
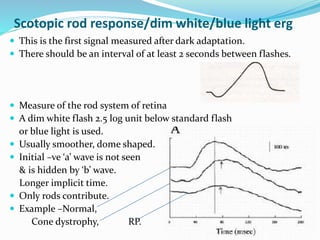
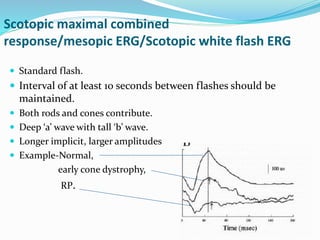
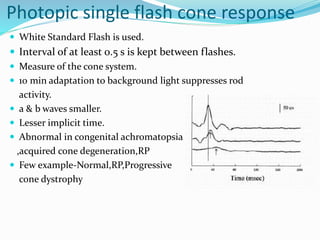
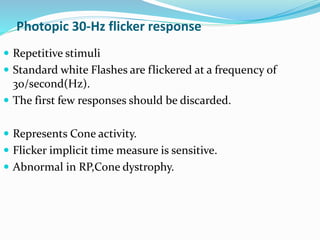
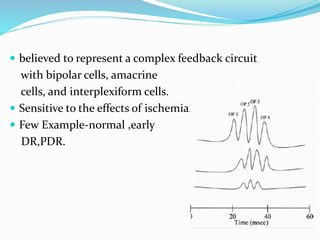
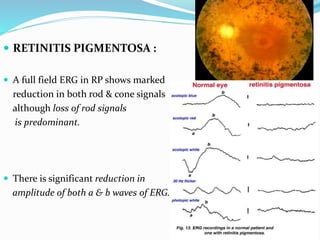
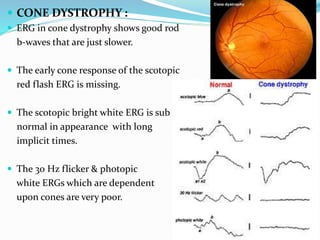
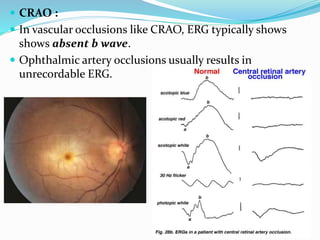
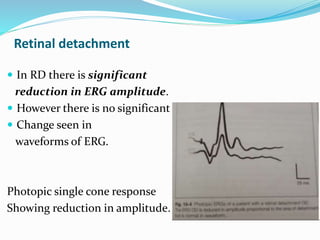
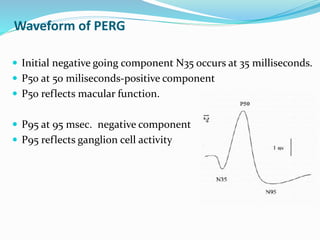
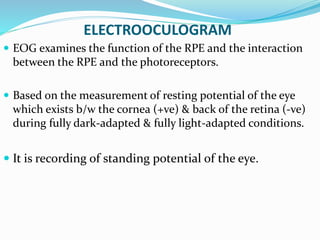
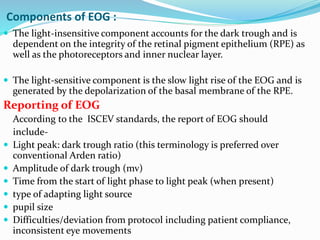
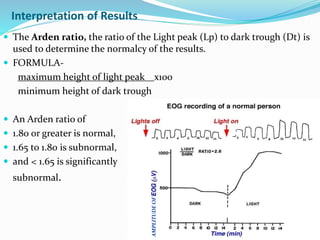
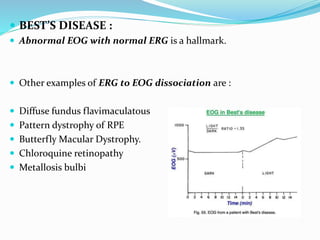
This document provides an overview of electroretinography (ERG), including:
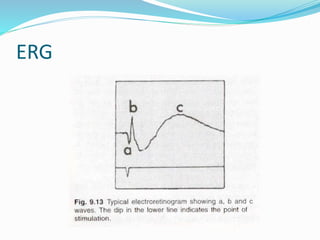
- The three main waves that make up the ERG (a, b, c waves) and their origins
- Protocols for performing ERG based on ISCEV standards
- Clinical applications of ERG for diagnosing and monitoring various retinal conditions like retinitis pigmentosa, cone dystrophy, retinal detachment, and more.
- Interpretation of normal and abnormal ERG responses.