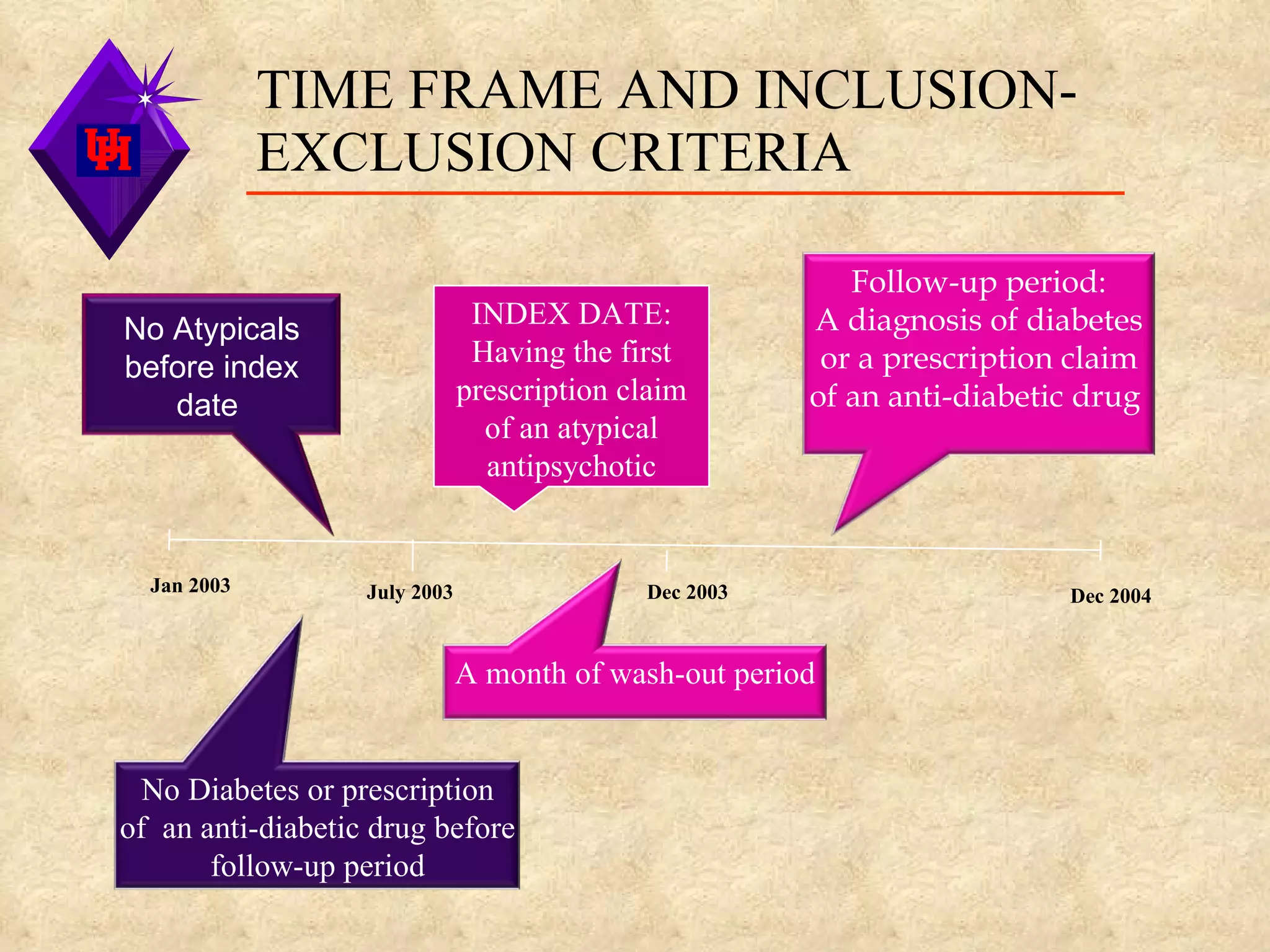
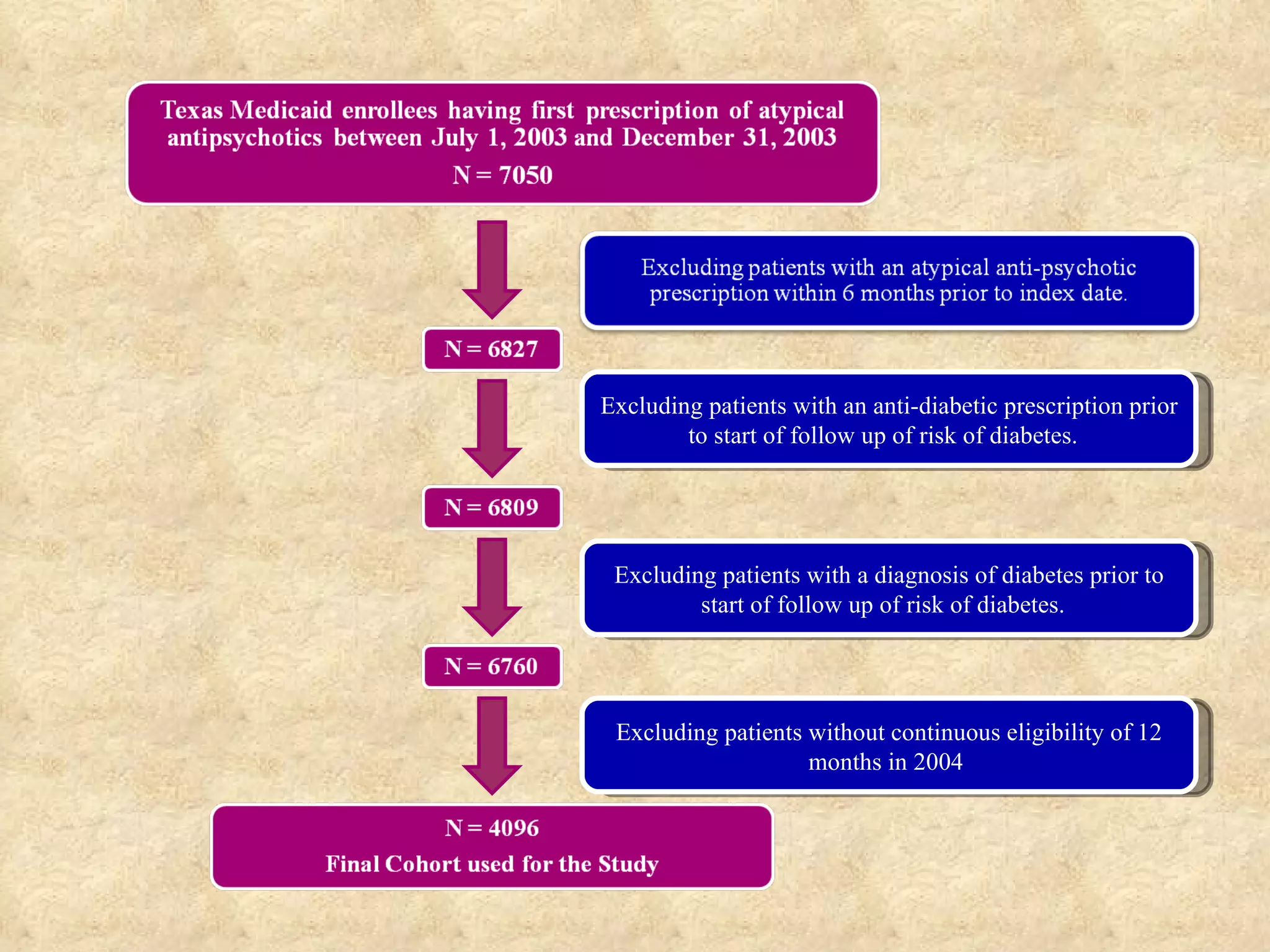
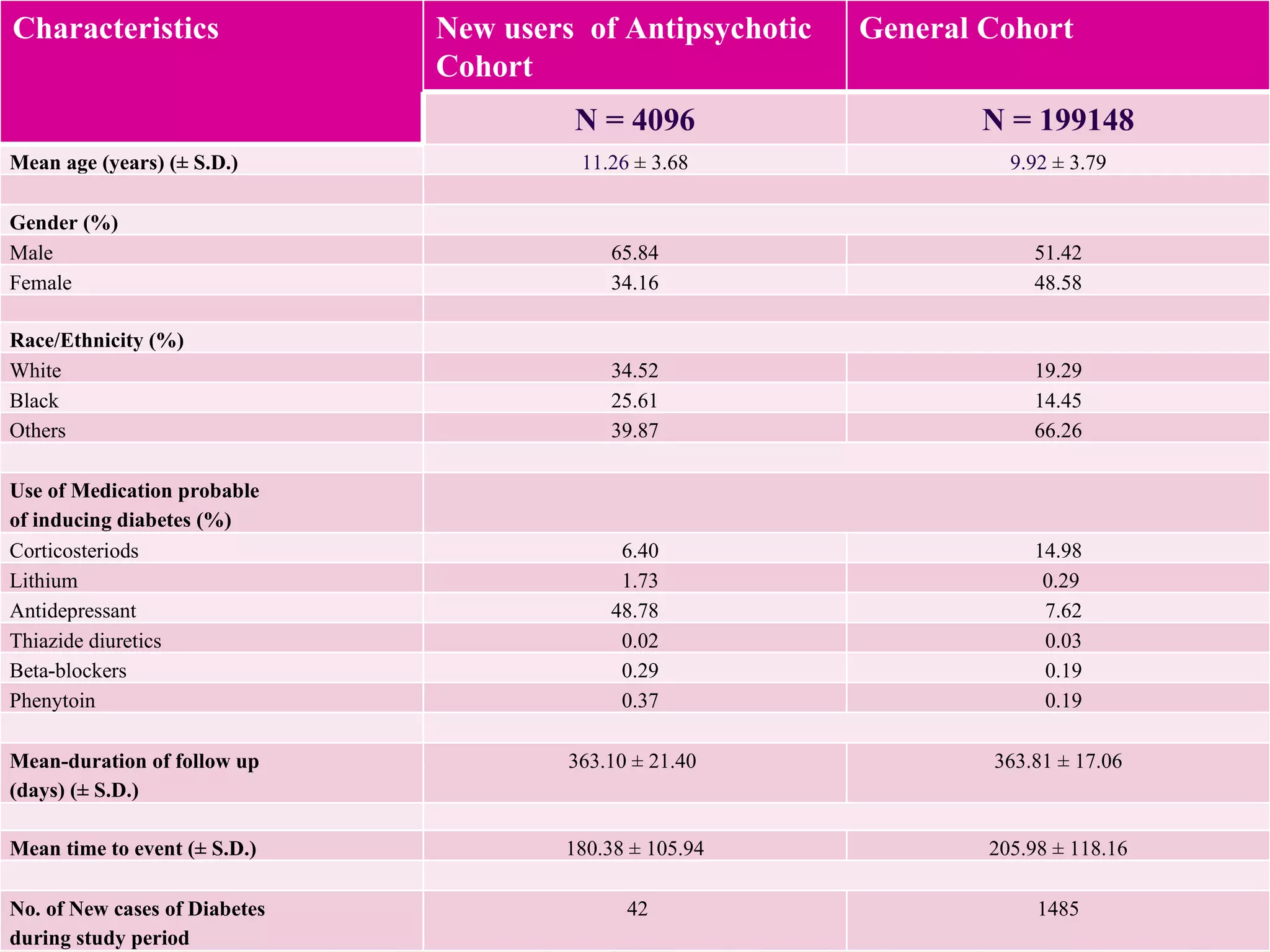
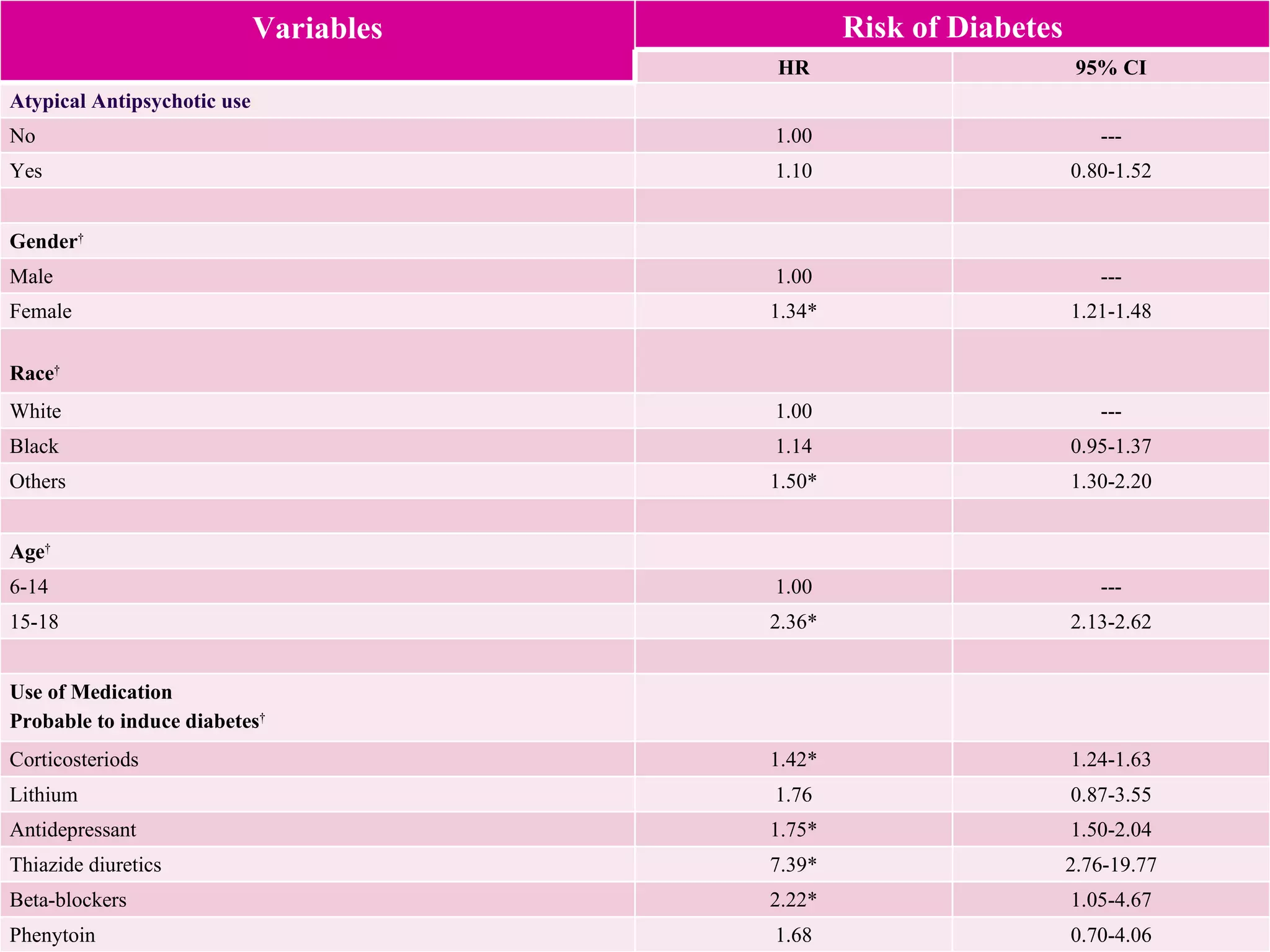
This study examined the risk of developing diabetes among children and adolescents aged 6 to 20 years old who were prescribed single atypical antipsychotic drugs using Texas Medicaid data. The study found no significant increase in risk of diabetes for children prescribed atypical antipsychotics compared to non-users. However, the results may not be generalizable due to limitations such as not accounting for dosage, BMI, glucose levels, or family history of diabetes which could influence risk. Larger and longer term studies are still needed to better understand the metabolic risks of atypical antipsychotics in pediatric populations.