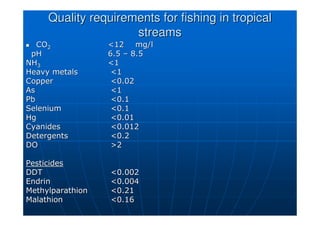
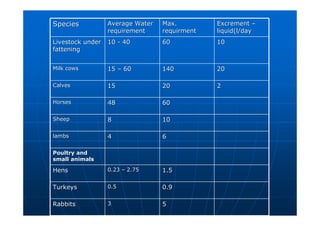
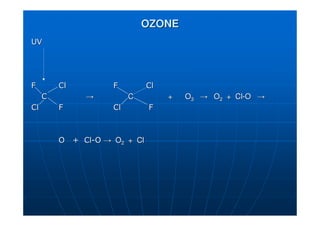
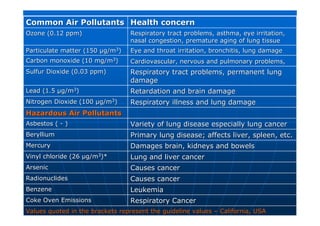
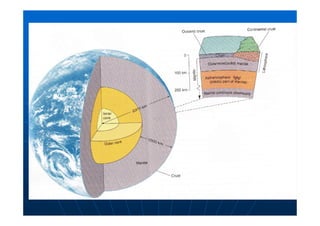
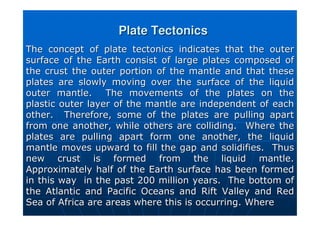
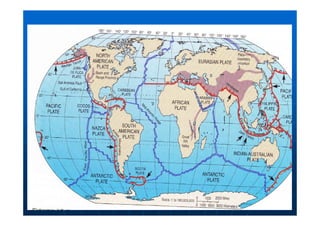
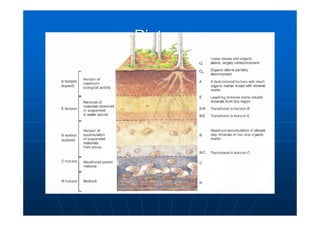
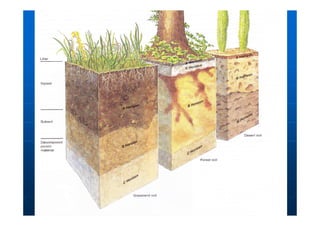
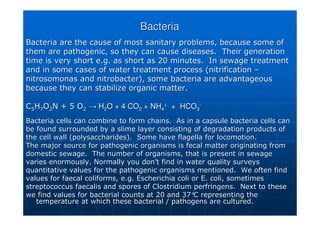
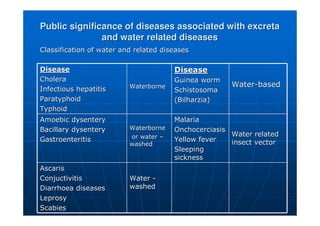
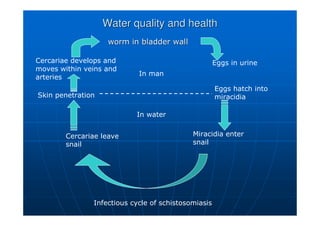
This document contains lecture notes on environmental science from Dr. R. Buamah. It defines environmental science as an interdisciplinary field involving both scientific and social aspects of human impact on the world. It also discusses concepts like ecosystems, which involve the movement of energy and materials through communities of organisms and their environment.


































































































![ARSENIC
ARSENIC
Long term exposure to drinking water with [As]
10 µg/l
Can cause cancer of the skin, lungs, urinary
bladder, kidney, etc
Skin pigmentation,
Hardening, thickening of skin and laceration of
sole of feet.
Retardation in the intelligence of children.
Nausea, vomiting, stomach pain, numbness of
hands and feet etc.
In Ghana – upsurge in the incidence of cancer
especially breast, meanwhile patronage
groundwater as drinking water source is on the
increase.](https://image.slidesharecdn.com/environmentalstudies-ce1552-240219000247-0f010828/85/Environmental-Studies-CE-155-2-pdf-by-117-320.jpg)
![ARSENIC
ARSENIC
Long term exposure to drinking water
with [As] 10 µg/l
Can cause cancer of the skin, lungs,
urinary bladder, kidney, etc
Skin pigmentation,
Hardening and laceration of sole of feet.
Retardation in the intelligence of children.
In Ghana – upsurge in the incidence of
cancer especially breast, meanwhile
patronage groundwater as drinking water
source is on the increase.](https://image.slidesharecdn.com/environmentalstudies-ce1552-240219000247-0f010828/85/Environmental-Studies-CE-155-2-pdf-by-118-320.jpg)
![FLUORIDE
FLUORIDE
Inorganic anion
Inorganic anion
Allerged
Allerged to be impact benefits to humans.
to be impact benefits to humans.
Recent finds
Recent finds –
– controversy
controversy
WHO
WHO –
– Europe
Europe –
– Rate of decrease of cavities in teeth of
Rate of decrease of cavities in teeth of
childn
childn in regions with / without
in regions with / without –
– same.
same.
The claim of F
The claim of F-
-
being beneficial has not held up
being beneficial has not held up
independent scientific scrutiny.
independent scientific scrutiny.
High [F
High [F-
-
]
] →
→ more brittle and fragile bones, increased bone mass
more brittle and fragile bones, increased bone mass
density but reduce strength of the bone at the same time due to
density but reduce strength of the bone at the same time due to
F
F-
- caused defects in the bone structure.
caused defects in the bone structure.
F
F-
- + Al in drinking water
+ Al in drinking water →
→ accumulates in brain
accumulates in brain →
→ neurotoxic
neurotoxic
morphological changes.
morphological changes.
A study in China has shown that even accepted levels in F
A study in China has shown that even accepted levels in F-
- in water
in water
(0.88 mg/l) may affect children
(0.88 mg/l) may affect children’
’s intelligence
s intelligence
High F
High F-
- is also a contributing factor to a bone cancer condition of
is also a contributing factor to a bone cancer condition of
osteosarcoma
osteosarcoma.
.
May cause elevation in blood sugar possibly exacerbating diabete
May cause elevation in blood sugar possibly exacerbating diabetes.
s.](https://image.slidesharecdn.com/environmentalstudies-ce1552-240219000247-0f010828/85/Environmental-Studies-CE-155-2-pdf-by-119-320.jpg)













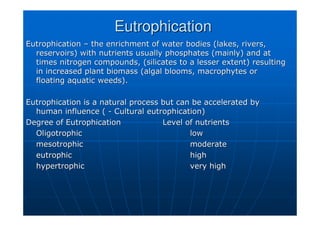
![Eutrophication
Eutrophication
Trophic
Trophic [P]
[P] [
[Chl
Chl]
] secchi
secchi
µ
µg/l
g/l µ
µg/l
g/l m
m
Oligo
Oligo 10
10 2.5
2.5 6
6
Meso
Meso 10
10 –
– 35
35 2.5
2.5 –
– 8
8 3
3 –
– 6
6
Eu
Eu 35
35 –
– 100
100 8
8 –
– 25
25 1.5
1.5 –
– 3
3
Hyper
Hyper 100
100 25
25 1.5
1.5](https://image.slidesharecdn.com/environmentalstudies-ce1552-240219000247-0f010828/85/Environmental-Studies-CE-155-2-pdf-by-137-320.jpg)
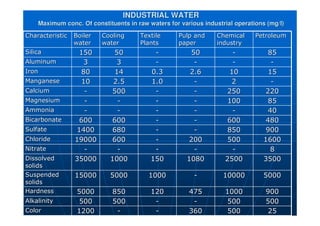

![Agriculture Water
Agriculture Water
Water Quality for irrigation (tropical conditions)
Water Quality for irrigation (tropical conditions)
Boron
Boron 1.25 mg/l
1.25 mg/l -
- sensitive crops
sensitive crops
4 mg/l
4 mg/l –
– tolerant crops
tolerant crops
Coliforms
Coliforms 100 per 100 ml if water is to be used for
100 per 100 ml if water is to be used for
unrestricted irrigation
unrestricted irrigation
SAR (
SAR (meq/l
meq/l)=
)= [Na
[Na+
+]
]
[Ca
[Ca2+
2+] + [Mg
] + [Mg2+
2+]
]
2
2
The porosity of Na
The porosity of Na –
– clay is lower than for Ca
clay is lower than for Ca –
– clay. This
clay. This
means that the permeability for air and water is lower.
means that the permeability for air and water is lower.](https://image.slidesharecdn.com/environmentalstudies-ce1552-240219000247-0f010828/85/Environmental-Studies-CE-155-2-pdf-by-140-320.jpg)