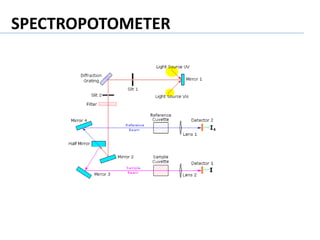
This document provides a summary of various environmental lab instruments and their applications. It lists instruments such as pH meters, analytical balances, turbidity meters, COD digesters, muffle furnaces, flame photometers, BOD incubators, water baths, hot air ovens, centrifuges, spectrophotometers, conductivity meters, and gas chromatographs. For each instrument, it briefly describes its purpose and common uses in environmental analysis and laboratory experimentation.