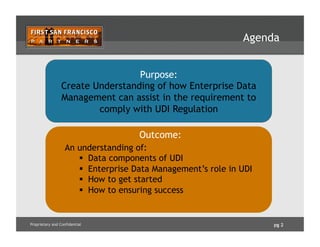
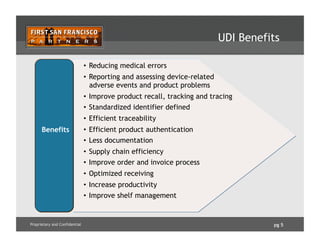
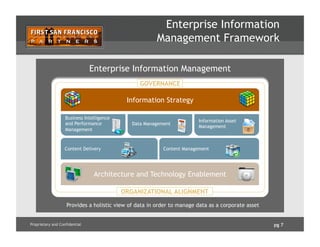
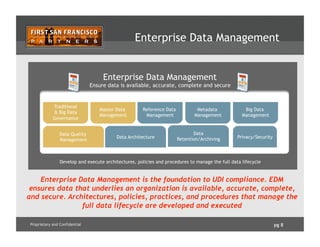
The document outlines how enterprise data management (EDM) aids in complying with Unique Device Identification (UDI) regulations for medical devices. It details the benefits of UDI, challenges with current data management, and emphasizes the need for data governance, quality, and a Product Information Management (PIM) hub to centralize product data. Successful implementation strategies and roles of stakeholders in ensuring compliance and data accuracy are also discussed.



![pg 6Proprietary and Confidential
[ ENTERPRISE DATA MANAGEMENT ]](https://image.slidesharecdn.com/enterprisedatamanagementenablesuniquedeviceidentificationudi-130710133049-phpapp01/85/Enterprise-Data-Management-Enables-Unique-Device-Identification-UDI-6-320.jpg)
![pg 9Proprietary and Confidential
[ DATA GOVERNANCE ]](https://image.slidesharecdn.com/enterprisedatamanagementenablesuniquedeviceidentificationudi-130710133049-phpapp01/85/Enterprise-Data-Management-Enables-Unique-Device-Identification-UDI-9-320.jpg)

![pg 14Proprietary and Confidential
[ DATA QUALITY ]](https://image.slidesharecdn.com/enterprisedatamanagementenablesuniquedeviceidentificationudi-130710133049-phpapp01/85/Enterprise-Data-Management-Enables-Unique-Device-Identification-UDI-14-320.jpg)



![pg 19Proprietary and Confidential
[ MASTER DATA MANAGEMENT ]](https://image.slidesharecdn.com/enterprisedatamanagementenablesuniquedeviceidentificationudi-130710133049-phpapp01/85/Enterprise-Data-Management-Enables-Unique-Device-Identification-UDI-19-320.jpg)

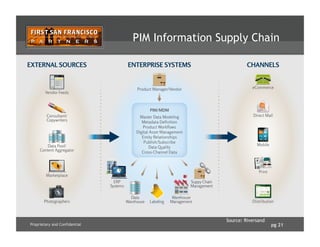

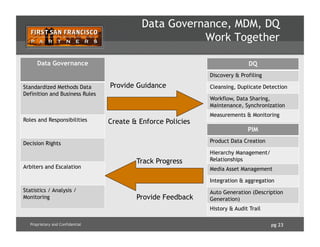
![pg 24Proprietary and Confidential
[ GETTING STARTED ]](https://image.slidesharecdn.com/enterprisedatamanagementenablesuniquedeviceidentificationudi-130710133049-phpapp01/85/Enterprise-Data-Management-Enables-Unique-Device-Identification-UDI-24-320.jpg)

![pg 26Proprietary and Confidential
[ ENSURING SUCCESS ]](https://image.slidesharecdn.com/enterprisedatamanagementenablesuniquedeviceidentificationudi-130710133049-phpapp01/85/Enterprise-Data-Management-Enables-Unique-Device-Identification-UDI-26-320.jpg)
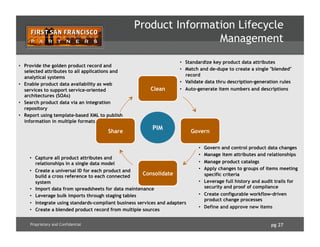

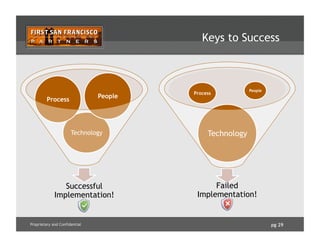

![pg 31Proprietary and Confidential
[ SECTION TITLE ]
Proprietary & Confidential
[ QUESTIONS? ]](https://image.slidesharecdn.com/enterprisedatamanagementenablesuniquedeviceidentificationudi-130710133049-phpapp01/85/Enterprise-Data-Management-Enables-Unique-Device-Identification-UDI-31-320.jpg)