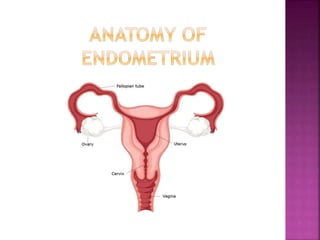
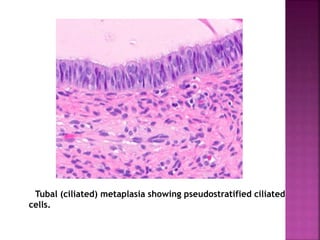
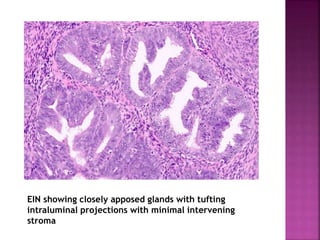
The document discusses the histology and histopathology of the endometrium. It begins by describing the normal histology and changes that occur throughout the menstrual cycle, including the proliferative, secretory, and menstrual phases. It then discusses gestational changes, effects of contraceptives and hormone replacement therapy. The document also covers endometritis, dysfunctional uterine bleeding, and endometriosis - their histopathological features, causes, and theories of pathogenesis.